What Is High-Risk Mantle Cell Lymphoma, and How Should We Treat It?
Improving patient prognostication in mantle cell lymphoma informs treatment decisions and enhances our understanding of the disease's biology.
blood cells : © Dr_Microbe - stock.adobe.com
Soon after mantle cell lymphoma (MCL) was recognized as a distinct subtype of B-cell non-Hodgkin lymphoma in 1992, researchers began to investigate the clinical outcomes of patients, noting the wide spectrum of outcomes that we still observe today. Refining our ability to prognosticate patients with MCL has been a major effort all the while using such information to inform treatment decisions and understanding the underlying disease biology of MCL.
Defining High-Risk MCL
We consider risk stratification within MCL based on clinical, pathologic, and molecular grounds. Patients with high-risk MCL typically present with a more rapid onset of symptoms and are frequently ill at diagnosis. The Mantle Cell International Prognostic Index (MIPI; age, lactate dehydrogenase, performance status, and leukocyte count) is a clinical scoring system and was shown to be prognostic in the up-front setting regardless of therapeutic approach.1 According to MIPI, the median overall survival (OS) was 29 months in high-risk patients, 51 months for those who were intermediate risk, and was not reached for low-risk patients.
Many pathologic features are characteristic of high-risk MCL. In particular, the percentage of tumor cells staining for Ki67 correlates with aggressive behavior and shortened survival in numerous studies.2 The combined MIPI incorporates Ki67 (< 30% vs ≥ 30%) and separated patients into 4 risk groups with 5-year OS rates of 85%, 72%, 43%, and 17%.3 Blastoid or pleomorphic histology4 is also associated with poor outcomes, as is tumor p53 overexpression.5
The most established high-risk genomic features include complex karyotype6 and TP53 aberrancy.7 Other genomic aberrations have also been associated with shortened survival, such as ATM alterations or CDKN2A/B deletions.8 The Nordic MCL2 study (ISRCTN87866680)7 established the inferior outcomes with TP53-aberrant patients undergoing high-dose therapy plus autologous stem cell rescue (HDT/ASCR). Herein, among 170 patients, those with mutated TP53 had a median progression-free survival (PFS) of 0.9 years vs 10.2 years for TP53-unmutated patients. Therefore, many eschew HDT/ASCR in patients with TP53-mutated MCL, and we follow this practice.
Treating High-Risk MCL
The optimal up-front treatment for patients with high-risk MCL is not fully defined, is evolving over time, and is individualized based on each patient’s clinical status, age, comorbidities, and goals of care.
Up-front Chemotherapy-Based Approaches
The TRIANGLE study (NCT02858258), which was presented in 2022,9 established a new up-front standard of care for patients with MCL who are eligible for HDT/ASCR. This study compared induction immunochemotherapy plus HDT/ASCR vs this approach plus ibrutinib (Imbruvica; during induction and maintenance) vs immunochemotherapy plus ibrutinib, followed by ibrutinib maintenance without HDT/ ASCR. Among 870 patients, only 15% were MIPI high risk. The ibrutinib- containing arms showed a benefit, and this effect was maintained even in high-risk patients (p53 overexpression > 50% or Ki67 ≥ 30%). Thus, TRIANGLE is a viable frontline treatment option for fit patients with high-risk MCL.
Chemotherapy-Free Approaches
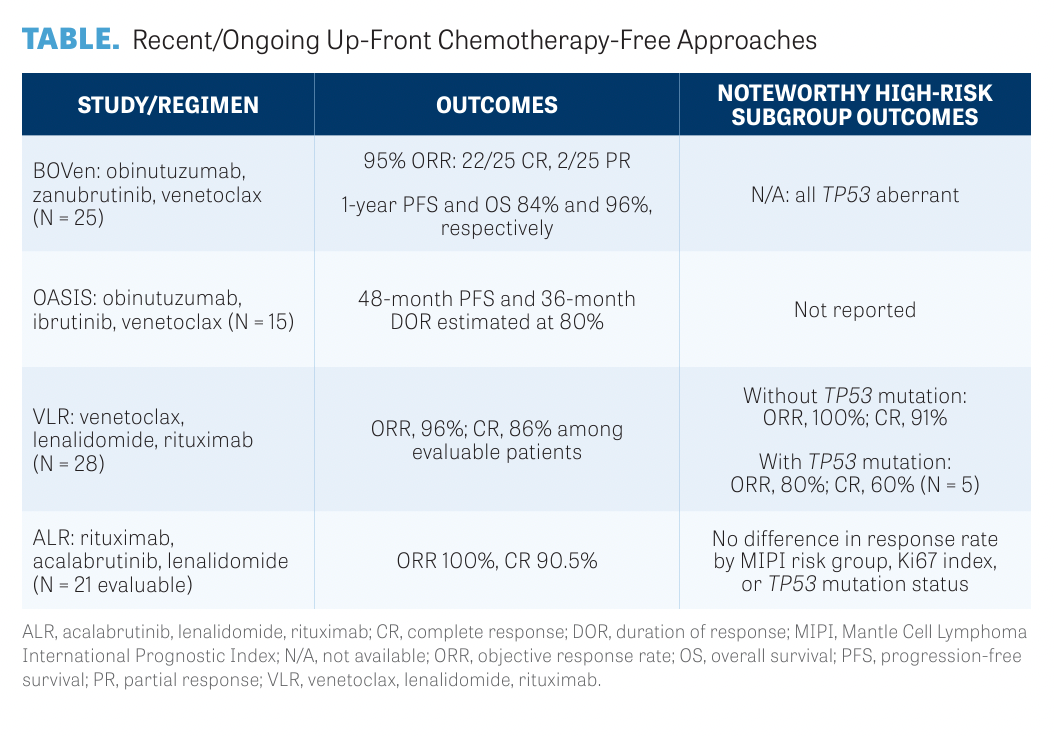
Numerous studies have investigated up-front chemotherapy-free treatment regimens (TABLE),10-13 including the multicenter BOVen study (NCT03824483; zanubrutinib [Brukinsa], obinutuzumab [Gazyva], and venetoclax [Venclexta]),10,14 the OASIS study (NCT02419560; obinutuzumab, venetoclax, and ibrutinib), the VLR study (NCT03523975; venetoclax, lenalidomide [Revlimid], and rituximab [Rituxan]),11 and the ALR study (NCT03863184; acalabrutinib [Calquence], lenalidomide, and rituximab).12
The BOVen study solely enrolled patients with TP53 aberrancy, whereas patients in the ALR trial (n = 24) were 21% MIPI high risk, 29% had TP53-mutated tumors, and 37% had Ki67 greater than or equal to 30%.
Patients in VLR (n = 29) were a more high-risk population: 88% were MIPI high risk, 7% had blastic or pleomorphic histology, 2 had TP53-mutated tumors, and 5 had TP53 deletions.
Finally, in OASIS (n = 15), 55% of patients were MIPI high risk, 1 (7%) patient had pleomorphic histology, 2 (13%) patients had TP53-mutated tumors, and 6 (40%) patients had 17p deletions.
Although early promising results are seen, longer follow-up is needed.
Relapsed/Refractory High-Risk MCL
CD19-directed chimeric antigen receptor (CAR) T-cell therapy (brexucabtagene autoleucel [brexu-cel] and lisocabtagene maraleucel [liso-cel]) is an efficacious treatment approach for relapsed/refractory (R/R) high-risk MCL.
Recently updated data (median follow-up, 35.6 months) from the ZUMA-2 study (NCT02601313; n = 68)15 of brexu-cel showed noteworthy overall response rates (ORRs): complete response rate (CRR) of 100% for 6 patients with TP53-mutated MCL, an 82% ORR and 53% CRR for 17 patients with blastoid MCL, and an 89% ORR and 76% CRR for 37 patients whose MCL who had a Ki67 of greater than or equal to 50%.
Only 2 patients with TP53- mutated MCL and 4 patients with blastoid MCL were still in remission at time of publication. A large consortium study of real-world data for brexu-cel use showed a lower CRR (72% vs 88%) for patients with TP53-mutated MCL.16
Liso-cel was studied in 32 patients,17 many of whom had high-risk features: 37.5% with blastoid morphology, 72% with Ki67 of greater than or equal to 30%, and 22% with TP53- mutated MCL.
Consolidative Allogeneic HCT
For patients with high-risk MCL and/or patients with R/R MCL, we often consider consolidative allogeneic hematopoietic cell transplantation if another remission is attained and there is a suitable donor identified. Long-term follow-up data17 suggest this approach has curative potential. Despite encouraging advancements, there remains a number of unanswered questions in high-risk MCL:
1. Are there molecular subgroupings within high-risk MCL that can further refine prognostication to tailor treatments?
2. How can we build on the success of the TRIANGLE study in future efforts?
3. Are chemotherapy-free regimens safe and effective in the frontline treatment of high-risk MCL? Are specific subgroups particularly suitable?
4. What is the role, if any, for up-front CAR T-cell therapy for treating high-risk MCL? This is the design for the CARMEN study (NCT01880567) of rituximab-ibrutinib (R-ibrutinib) followed by CAR T-cell therapy vs R-ibrutinib plus chemotherapy from the European MCL Network. Additionally, what is the durability of responses to CAR T in R/R MCL, and how will these data compare with outcomes with bispecific antibodies?
Concerted, multicenter, collaborative efforts are needed to urgently address these questions and to ultimately help patients.
Zachary Epstein-Peterson, MD, is a lymphoma specialist at Memorial Sloan Kettering Cancer Center. Anita Kumar, MD, is a lymphoma specialist at Memorial Sloan Kettering Cancer Center
REFERENCES
1. Hoster E, Dreyling M, Unterhalt M, Hasford J, Hiddemann W. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2006;108(11):814. doi:10.1182/blood.V108.11.814.814
2. Tiemann M, Schrader C, Klapper W, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol. 2005;131(1):29-38. doi:10.1111/j.1365-2141.2005.05716.x
3. Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol. 2014;32(13):1338-1346. doi:10.1200/JCO.2013.52.2466
4. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394. doi:10.1200/JCO.2015.63.8387
5. Nordström L, Sernbo S, Eden P, et al. SOX11 and TP53 add prognostic information to MIPI in a homogenously treated cohort of mantle cell lymphoma--a Nordic Lymphoma Group study. Br J Haematol. 2014;166(1):98-108. doi:10.1111/bjh.12854
6. Greenwell IB, Staton AD, Lee MJ, et al. Complex karyotype in patients with mantle cell lymphoma predicts inferior survival and poor response to intensive induction therapy. Cancer. 2018;124(11):2306-2315. doi:10.1002/cncr.31328
7. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910. doi:10.1182/blood-2017-04-779736
8. Clot G, Jares P, Giné E, et al. A gene signature that distinguishes conventional and leukemic nonnodal mantle cell lymphoma helps predict outcome. Blood. 2018;132(4):413-422. doi:10.1182/blood-2018-03-838136
9. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized triangle trial by the European MCL Network. Blood. 2022;140(suppl 1):1-3. doi:10.1182/blood-2022-163018
10. Kumar A, Soumerai JD, Abramson JS, et al. Preliminary safety and efficacy from a multicenter, investigator-initiated phase II study in untreated TP53 mutant mantle cell lymphoma with zanubrutinib, obinutuzumab, and venetoclax (BOVen). Blood. 2021;138(suppl 1):3540. doi:10.1182/blood-2021-151831
11. Phillips TJ, Bond D, Takiar R, et al. Adding venetoclax to lenalidomide and rituximab is safe and effective in patients with untreated mantle cell lymphoma. Blood Adv. 2023;7(16):4518-4527. doi:10.1182/bloodadvances.2023009992
12. Ruan J, Leonard JP, Chen GZ, et al. Phase 2 trial of acalabrutinib-lenalidomide-rituximab (ALR) with real-time monitoring of MRD in patients with treatment-naïve mantle cell lymphoma. Blood. 2022;140(suppl 1):175-177. doi:10.1182/blood-2022-158656
13. Le Gouill S, Morschhauser F, Chiron D, et al. Long term follow-up of untreated/relapsing MCL patients with the ibrutinib, obinutuzumab, and venetoclax combination. HemaSphere. 2023;7(suppl 3):e6048802. doi:10.1097/01.HS9.0000971256.60488.02
14. Kumar A, Soumerai J, Abramson JS, et al. A multicenter phase 2 trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in patients with treatment-naïve, TP53-mutant mantle cell lymphoma. Blood. 2023;142(suppl 1):738. doi:10.1182/blood-2023-180069
15. Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023;41(14):2594-2606. doi:10.1200/JCO.22.01797
16. Palomba ML, Gordon LI, Siddiqi T, et al. Safety and preliminary efficacy in patients with relapsed/refractory mantle cell lymphoma receiving lisocabtagene maraleucel in TRANSCEND NHL 001. Blood. 2020;136(suppl 1):10-11. doi:10.1182/blood-2020-136158
17. Lin RJ, Ho C, Hilden PD, et al. Allogeneic haematopoietic cell transplantation impacts on outcomes of mantle cell lymphoma with TP53 alterations. Br J Haematol. 2019;184(6):1006-1010.
doi:10.1111/bjh.15721
Leon-Ferre Explores Targeting of PIK3CA Alterations in ER+ Breast Cancer
July 24th 2024During a live Community Case Forum event in partnership with the Minnesota Society of Clinical Oncology, Roberto A. Leon-Ferre, MD, discussed drugs targeting PIK3CA alterations in patients with ER+ metastatic breast cancer.
Read More
Roundtable Roundup: Treatment for Metastatic pMMR Endometrial Cancer
July 23rd 2024In separate, live virtual events, Michael J. Birrer, MD, PhD, and Jubilee Brown, MD, surveyed participants on the treatment of a postmenopausal woman with stage IVA endometrial cancer after first-line chemotherapy.
Read More
George Explores Impact of Risk Status With Cabozantinib/Nivolumab in Advanced RCC
July 19th 2024During a Case-Based Roundtable® event, Daniel George, MD, discussed the results of the CheckMate 9ER trial across favorable, intermediate, and poor risk groups in patients with advanced renal cell carcinoma.
Read More
Depth of Response With Quadruplet Regimens Considered in Newly Diagnosed Multiple Myeloma
July 18th 2024During a Case-Based Roundtable® event, Timothy Schmidt, MD, and participants discussed treatment selection for a 54-year-old patient with transplant eligible R-ISS stage 2/R2-ISS stage 3 IgG-κ myeloma.
Read More
Rossetti Reviews Myelofibrosis Risk Stratification and Outcome Data for Pacritinib
July 17th 2024During a Case-Based Roundtable® event, James M. Rossetti, DO, discussed the role of risk scoring and stratification tools and treatment for a patient with declining hemoglobin and platelet counts due to primary myelofibrosis.
Read More