Neoadjuvant Use of ICB in NSCLC Gains Favor
Findings from recent clinical research have firmly entrenched the use of neoadjuvant immune checkpoint blockade in the treatment of early-stage non-small cell lung cancer.

Findings from recent clinical research have firmly entrenched the use of neoadjuvant immune checkpoint blockade (ICB) in the treatment of early-stage non-small cell lung cancer (NSCLC). Historically, chemotherapy was given either before or after surgery in this setting, yielding only a modest benefit in survival.
“As immune checkpoint blockade emerged in the metastatic setting, there was increased interest in bringing these agents into earlier settings,” Samuel Rosner, MD, an associate professor at the University of Maryland School of Medicine in Baltimore, said during a presentation1 at the 21st Annual Winter Lung Cancer Conference®, February 2 to 4, 2024, in Hollywood, Florida, sponsored by Physicians’ Education Resource®, LLC (PER®).
The clinical impact of this rationale was observed in recent trials, including CheckMate 816 (NCT02998528; Visual Synopsis),2 KEYNOTE-671 (NCT03425643),3 and others.
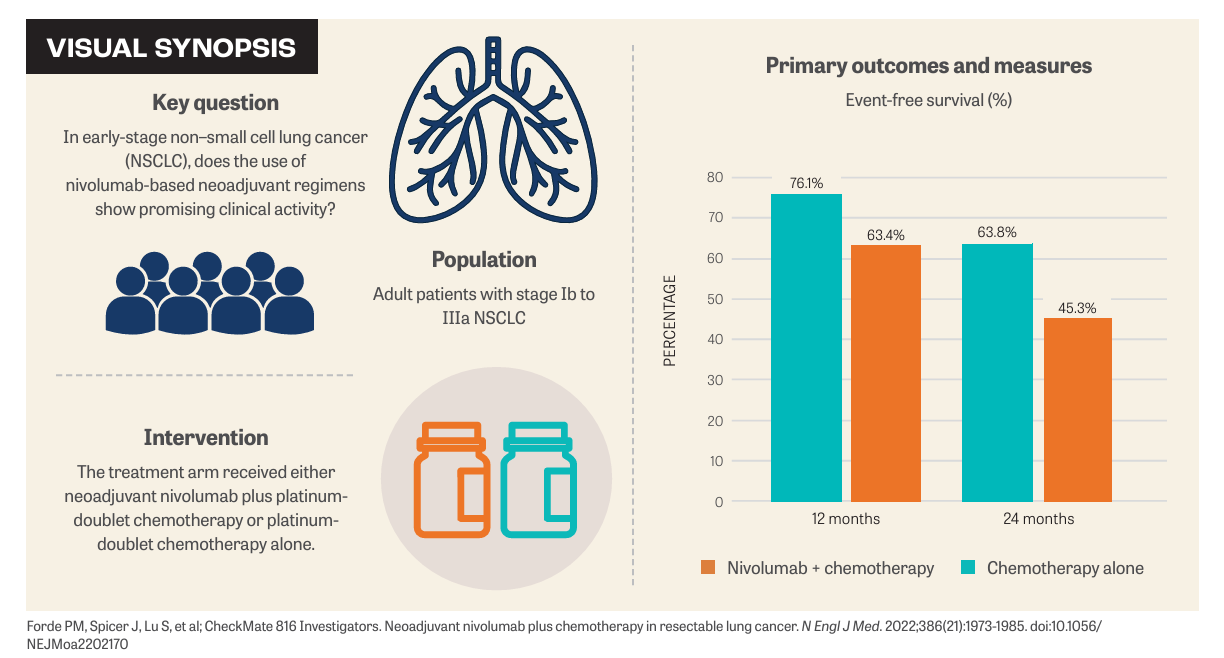
The phase 3 CheckMate 816 trial randomly assigned 358 patients with stage Ib to IIIa NSCLC to receive either nivolumab (Opdivo) plus platinum-based chemotherapy or platinum-based chemotherapy alone, followed by surgery. The primary end points were event-free survival (EFS) and pathological complete response (pCR). The secondary end point was overall survival (OS).2
Across most subgroups, EFS and the pCR rate favored the treatment arm. The median EFS for the nivolumab plus chemotherapy arm was 31.6 months (95% CI, 30.2-not reached [NR]) compared with 20.8 months (95% CI, 14.0-26.7) in the chemotherapy-alone arm (HR, 0.63; 97.38% CI, 0.43-0.91; P = .005). The percentage of patients with pCR was 24.0% (95% CI, 18.0%-31.0%) and 2.2% (95% CI, 0.6%-5.6%), respectively (odds ratio, 13.94; 99% CI, 3.49-55.75; P < .001).2
“In general, all subgroups derived some degree of benefit from the treatment, although there were some exceptions—those patients with stage III disease, patients with nonsquamous histology, and patients with PD-L1–positive disease,” Rosner said.
Rosner noted that one of the initial concerns with introducing checkpoint inhibitors earlier on in the treatment regimen was that it could lead to complications or delays in surgery. “But based on the data from CheckMate 816, the opposite was noted in the nivolumab-treated group,” Rosner said. “Eighty-three percent of patients in the treatment group were more likely to undergo definitive resection.” He added that incidences of immune-related adverse events were also low, with many of them low grade, and did not contribute significantly to cancellations or delays in surgery.
The phase 3 KEYNOTE-671 trial took a perioperative approach that included both neoadjuvant and adjuvant immune checkpoint inhibition. Patients with stage II, IIIa, or IIIb NSCLC were randomly assigned to receive neoadjuvant pembrolizumab (Keytruda) plus cisplatin-based chemotherapy followed by surgery and adjuvant pembrolizumab (n = 397) vs placebo plus cisplatin-based chemotherapy followed by surgery and adjuvant pembrolizumab (n = 400). Primary end points were EFS and OS. Secondary end points were major pathological response, pCR, and safety.3
The EFS rate at 24 months was 62.4% in the pembrolizumab group and 40.6% in the placebo group (HR, 0.58; 95% CI, 0.46-0.72; P < .001). The OS rate at 24 months was 80.9% in the pembrolizumab group and 77.6% in the placebo group, although this was not significant (P = .02). Researchers observed a major pathological response rate of 30.2% in the pembrolizumab group and 11.0% in the placebo group (difference = 19.2 percentage points; 95% CI, 13.9-24.7; P < .0001) and a pCR rate of 18.1% and 4.0%, respectively (difference = 14.2 percentage points; 95% CI, 10.1-18.7; P < .0001).
“All subgroups derived some benefit from the addition of perioperative pembrolizumab and, in particular, patients with squamous cell histology did quite well with this regimen,” Rosner said.
Other trials showing the benefit of perioperative ICB include the AEGEAN trial (NCT03800134) and CheckMate 77T (NCT04025879).4,5 When looking at the data from these trials overall, Rosner offered some main observations.
“About 15% to 20% of patients are sadly not making it to surgery, primarily because of primary progression of the patient’s cancer. On the other hand, about 20% to 25% of patients are having robust response to treatment,” Rosner said. “CheckMate 816 and KEYNOTE-671 used a cisplatin-only regimen. That’s important when we’re thinking about patient selection,” Rosner continued.
Biomarkers
One major benefit from these trials is the emergence of biomarkers, including the depth of pathological response, Rosner said. “It seems to carry a prognostic impact for these patients, who seem to do quite well in the postoperative setting with regards to EFS and OS,” he said. “In particular, PD-L1 expression seems to have both an important predictive as well as prognostic property that can be leveraged,” Rosner continued. “Patients who have positive PD-L1 expression seem to derive more benefit.” He noted that patients with PD-L1–negative expression also derive some benefit, albeit to a lesser extent.
It is apparent that rapid and reliable biomarker testing is increasingly important in this setting. But a number of questions remain unanswered. For example, is perioperative treatment better than neoadjuvant and if so, for whom? Are there patients for whom surgery should be a priority? Should postresection management be based on pathological response? Should this approach be explored for borderline resectable disease?
“I think what’s needed is more clinical trials in this space [to gain further clarity] before adopting this approach in the clinical standard-of-care setting,” Rosner concluded.
REFERENCES:
1. Rosner S. Impact of perioperative/neoadjuvant therapy. Presented at: 21st Annual Winter Lung Cancer Conference; February 2-4, 2024; Hollywood, FL. Accessed February 8, 2024.
2. Forde PM, Spicer J, Lu S, et al; CheckMate 816 Investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
3. Wakelee H, Liberman M, Kato T, et al; KEYNOTE-671 Investigators. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. N Engl J Med. 2023;389(6):491-503. doi:10.1056/NEJMoa2302983
4. Heymach JV, Harpole D, Mitsudomi T, et al; AEGEAN Investigators. Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684. doi:10.1056/NEJMoa2304875
5. Cascone T, Awad MM, Spicer JD, et al. CheckMate 77T: phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. Ann Oncol. 2023;34(suppl 2):S1295. doi:10.1016/j.annonc.2023.10.050
THIO Shows Lower Toxicity Than Standard Treatments in NSCLC Study
July 25th 2024A phase 2 trial shows that THIO, a new investigational drug, combined with cemiplimab, may be an effective and well-tolerated treatment for patients with advanced non–small cell lung cancer who have exhausted other options.
Read More
Leon-Ferre Explores Targeting of PIK3CA Alterations in ER+ Breast Cancer
July 24th 2024During a live Community Case Forum event in partnership with the Minnesota Society of Clinical Oncology, Roberto A. Leon-Ferre, MD, discussed drugs targeting PIK3CA alterations in patients with ER+ metastatic breast cancer.
Read More
Roundtable Roundup: Treatment for Metastatic pMMR Endometrial Cancer
July 23rd 2024In separate, live virtual events, Michael J. Birrer, MD, PhD, and Jubilee Brown, MD, surveyed participants on the treatment of a postmenopausal woman with stage IVA endometrial cancer after first-line chemotherapy.
Read More