Scott Evaluates Treatment Options for Hydroxyurea-Resistant Polycythemia Vera
In a Community Case Forum event in partnership with the Washington State Medical Oncology Society, Bart Scott, MD, broke down various trials of hydroxyurea, ruxolitinib, and interferon in patients with polycythemia vera to assess outcomes such as hematocrit control and molecular response.
Bart Scott, MD
Professor, Transplantation Program
Clinical Research Division
Miklos Kohary and Natalia Zimonyi Kohary Endowed Chair
Fred Hutchinson Cancer Center
Seattle, WA

CASE SUMMARY
February 2018
- A 67-year-old man presented with frequent headache and dizziness.
- Prior medical history is notable for smoking.
- Physical exam: unremarkable, no splenomegaly
- Laboratory testing results:
- Hemoglobin, 20.5 g/dL
- White blood cell (WBC) count, 13,000/μL
- Platelet count, 380,000/μL
- Hematocrit, 68%
- The JAK2 V617F mutation was detected with a variant allele frequency of 65%.
- Bone marrow biopsy showed trilineage proliferation and pleomorphic megakaryocytes.
- The patient started phlebotomy, aspirin, and hydroxyurea (Hydrea).
- Over the next year the patient underwent several phlebotomies (phlebotomy dependent) and remained on aspirin and 2000 mg/day of hydroxyurea (note: maximum tolerated dose).
February 2019
- The patient had 2 phlebotomies since the last visit 3 months earlier.
- He is also experiencing fatigue and night sweats.
- Laboratory testing results:
- Hemoglobin, 15.5 g/dL
- WBC count, 13,000/μL
- Platelet count, 380,000/μL
- Hematocrit, 47%
Targeted Oncology: What is the significance of target hematocrit in patients with polycythemia vera (PV)?
SCOTT: In the CYTO-PV trial [NCT01645124], patients who were diagnosed with PV had to be over 18 years of age, and they could get any [treatment] they wanted, but the difference is [targeting] high hematocrit vs low hematocrit. The cytoreduction, which could include phlebotomy alone, was targeting hematocrit of less than 45%, or hematocrit between 45% to 50%.
Forty-one percent of the patients who received phlebotomy would have excursions to greater than 45%. You should keep in mind that saying your target hematocrit is less than 45% is vastly different from saying they always kept their hematocrit less than 45%. Those are 2 different things. [Researchers] have tried to extrapolate these data to mean something other than what it is…[and] it’s misleading.
The primary end point, which was thrombosis-free survival…was better in the low-hematocrit group than in the high-hematocrit group.1 Remember, all these patients were getting aspirin therapy. The end point was time until death from cardiovascular-related complications or thrombosis.
There were some interesting things that they found here. If your WBC count was over 11,000/μL, you’re at higher risk of thrombosis.2,3 Leukocytosis is a big driver of thrombosis, and phlebotomy is not an effective strategy for reducing WBC count. If you’ve got somebody with a WBC count of 15,000/μL and you’re treating with phlebotomy alone, maybe that’s not the best choice if they’re over the of 60. Most of these patients were on hydroxyurea. Some of them were managed with phlebotomy alone.
What trials support the use of ruxolitinib (Jakafi) in PV?
There have been 2 RESPONSE trials. The unique thing for RESPONSE-1 [NCT01243944] is that patients had to have an [enlarged] spleen to get into it. Some people have criticized RESPONSE-1, because we gave a Janus kinase inhibitor to patients with PV who had an [enlarged] spleen, and maybe they had some underlying fibrosis. Isn’t this just like the COMFORT I and II trials [NCT00952289; NCT00934544], and you’re selecting a group of patients with PV who are just worse to begin with? That’s why they did RESPONSE-2 [NCT02038036], which…[is] essentially the same, except in RESPONSE-2 patients didn’t have to have splenomegaly.
They were randomly assigned on a 1:1 basis to get ruxolitinib or standard therapy. Most patients who were getting standard therapy stayed on hydroxyurea. [However], these studies were for [patients who had] hydroxyurea resistance or intolerance. Why would investigators choose standard therapy with hydroxyurea? That tells you something about the definition of being resistant or intolerant to hydroxyurea.
The coprimary end points for RESPONSE-1 were spleen volume reduction and hematocrit control. For RESPONSE-2, they didn’t have [enlarged] spleens, so it was just hematocrit control. [In RESPONSE-1], the coprimary end points were met in the ruxolitinib group compared with hydroxyurea [Figure4]. That’s a [poor] control arm because we already knew hydroxyurea wasn’t working. It also met the secondary end point of symptom reduction as well, which is why ruxolitinib is approved for second line after hydroxyurea or intolerance. It’s a durable response…going out [to] 258 weeks.5
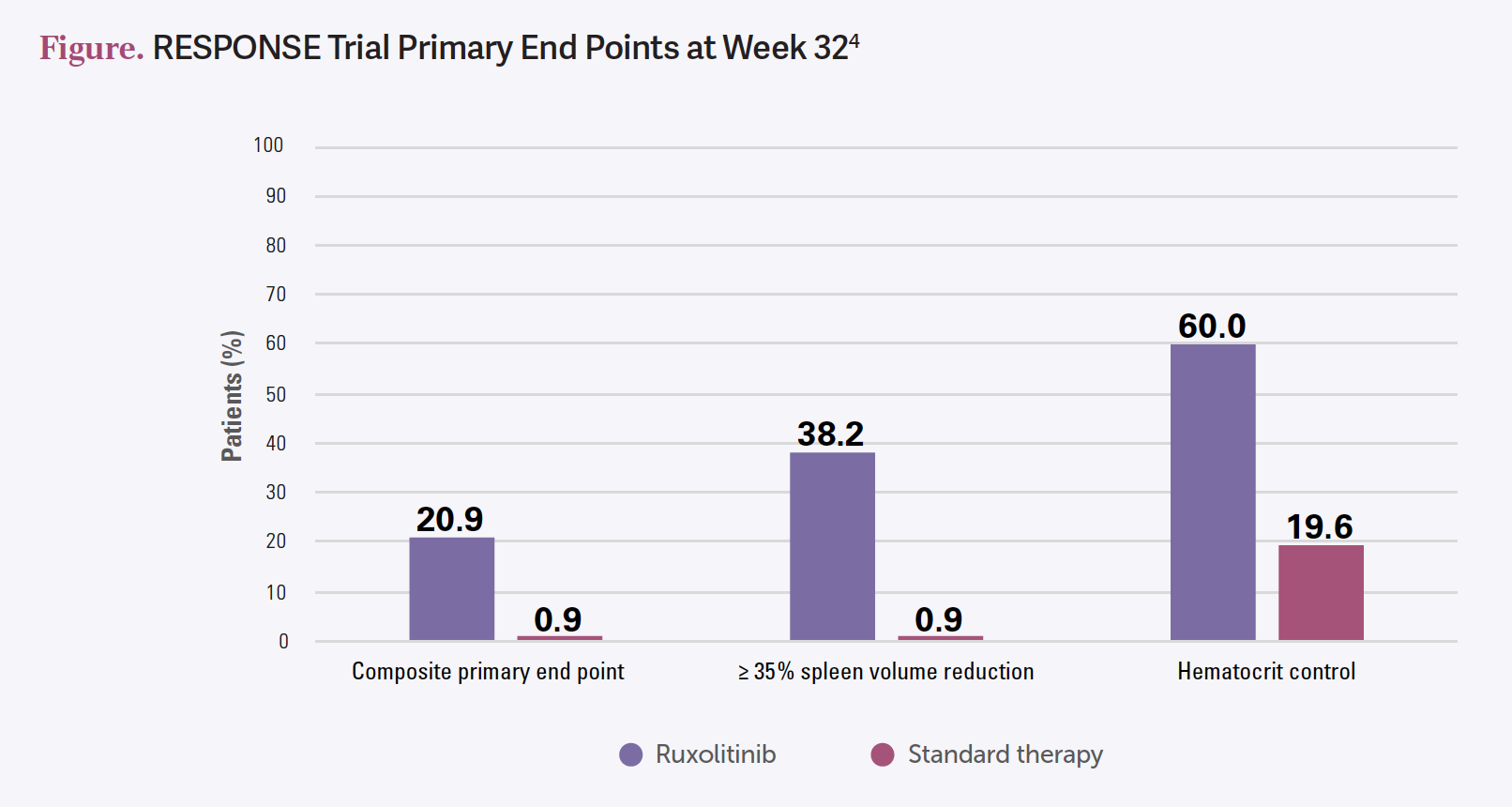
[Adverse events (AEs) included] anemia, thrombocytopenia, fatigue, and night sweats, and also the WBC count went down, which is good, because we just identified having a high WBC count as a risk factor.5,6 Ruxolitinib did reduce estimated thrombotic rates.
The MAJIC-PV study [ISRCTN61925716] was another randomized study, but in this case, [ruxolitinib] was compared with best available therapy for resistance or intolerance to hydroxyurea. It had similar design to RESPONSE-1 and RESPONSE-2. The primary outcome was complete response. [Looking at] the thrombotic events and bleeding events in the trial and the ruxolitinib arm compared with the best available therapy arm, the thrombotic events were decreased.7
The event-free survival [EFS] for ruxolitinib compared with best available therapy, with the EFS defined as thrombosis, bleeding, transformation, and death. Specifically for thrombosis, ruxolitinib reduced thrombotic rates in patients with PV. This was not an end point in the RESPONSE trials.
The MAJIC-PV trial was done specifically for this. Some people have questioned the ethics of doing this study when ruxolitinib was already approved, but this study was not done in the US. It was done in Europe, where the drug was not approved. When you look at this, approximately 75% of patients in the ruxolitinib arm had no thrombosis over 5 years [with an HR favoring ruxolitinib of 0.56; 95% CI, 0.32-1.00; P = .05].
What data support the use of interferon therapy for PV?
There have been 2 prospective randomized studies evaluating interferon therapy for PV. One was the MPN-RC 112 trial [NCT01258856], which I’m not [discussing]. The other one is the PROUD-PV study [NCT01949805], with pegylated interferon-alfa-2b [ropeginterferon; Besremi]. It was FDA approved [based on] phase 2 data. This is a subsequent phase 3 trial.
It included patients who are naive or hydroxyurea pretreated, and they were randomly assigned to receive either ropeginterferon or hydroxyurea. The primary end point was complete hematologic response with normal spleen size at 12 months.
The CONTINUATION-PV study [NCT02218047] was after crossover, and it just followed the [PROUD-PV] patients long term. What they saw is that the complete hematologic response rate was the same.8 This was a noninferiority study, so it wasn’t meant to show superiority.
The other thing is they looked at major molecular responses. You’re more likely to develop a major molecular response, defined as a 50% reduction in the variant allele frequency, at least of the driver mutation, which for most of these patients is going to be JAK2 V617F. Essentially, you’re more likely to develop a molecular remission if you’re treated with interferon compared with hydroxyurea, and the complete hematologic response rates are similar between the 2.8
Safety data [included] liver function test abnormalities reported for ropeginterferon [grade 3 or 4 gamma-glutamyltransferase elevation in 6% and alanine aminotransferase in 3%]. Treatment-related serious [AEs] were similar between the 2 [regimens].
Any [endocrine AE] occurred in 6% for ropeginterferon vs 2% in the control arm, [which was] mostly hydroxyurea. The hematocrit control was slightly better with interferon-based approaches over hydroxyurea.9 The [EFS] of ropeginterferon vs the control arm was statistically significantly different [EFS events in 5 of 95 patients with ropeginterferon vs 12 of 74 in the control arm at 6 or more years of treatment].
Does molecular response with interferon benefit patients, and is this an argument for interferon vs ruxolitinib?
There are no data to show that having a major molecular response is associated with an improved overall survival, and it would be almost impossible to do that study, because you’d have to follow them for 25 years. This is [an argument for interferon over ruxolitinib], but it’s a weak one, because it’s a surrogate end point. It’s easy to draw the conclusion that if we make your mutation go away, you’re going to do better. That seems fairly intuitive. But we’ve seen before in medicine when things that have been fairly intuitive haven’t proven ultimately to be true. We shouldn’t automatically say getting rid of your JAK2 [mutation] is going to make you live longer. We don’t know that.
What conclusions can be drawn from the Low-PV study (NCT03003325) of low-risk patients with PV?
The investigators of the CYTO-PV trial decided that they were going to do a study for low-risk PV and have as the end point hematocrit of less than 45%.10 I have an issue with that being the end point because the CYTO-PV study showed a better survival if the target hematocrit was less than 45%, but they didn’t show a better survival if the patient always kept it less than 45%. [They are] arguing that we should give even low-risk patients interferon because it’s going to keep patients at less than 45% more commonly with interferon than phlebotomy, but there are no data to show that that’s beneficial. This irritates me, particularly when you know that interferon makes patients sick…
I don’t [recommend] doing this. If you give interferon, you’re more likely to have better hematocrit control, but that’s a surrogate end point.10 That’s not a primary end point that I would be interested in from a patient perspective. In the CYTO-PV trial, with the patients who got phlebotomy, only [approximately] 40% cut their hematocrit [to] less than 45%.1 Did they have a worse survival? We didn’t analyze them separately, so we don’t know that. Having a target hematocrit of less than 45% is different from always [keeping] hematocrit less than 45%. For me, these are not compelling data.
There’s another reason to prefer interferon for a younger patient, and that is that it preserves fertility. If you have patients who are interested in [conceiving children], interferon should be preferred. The other thing I’ll say is that although hydroxyurea does not lead to leukemia, we know that based on patients who have an average age of 65. Taking someone who’s 65 and committing them to 10 years of hydroxyurea is different from taking someone 35 and committing them to 40 years of hydroxyurea. Duration of exposure may be important in risk of leukemia progression.
REFERENCES:
1. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/ NEJMoa1208500
2. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560- 561. doi:10.1182/blood-2015-04-638593
3. Tashi T. Hematocrit, white blood cells, and thrombotic events in the veteran population with polycythemia vera. Fed Pract. 2022;39(suppl 2):S43-S46. doi:10.12788/fp.0243
4. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426- 435. doi:10.1056/NEJMoa1409002
5.Kiladjian JJ, Zachee P, Hino M, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7(3):e226-e237. doi:10.1016/ S2352-3026(19)30207-8
6. Harrison CN, Griesshammer M, Miller C, et al. Comprehensive haematological control with ruxolitinib in patients with polycythaemia vera resistant to or intolerant of hydroxycarbamide. Br J Haematol. 2018;182(2):279-284. doi:10.1111/bjh.14764
7. Harrison CN, Nangalia J, Boucher R, et al. Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J Clin Oncol. 2023;41(19):3534-3544. doi:10.1200/JCO.22.01935
8. Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196-e208. doi:10.1016/S2352-3026(19)30236-4
9. Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b achieves patient-specific treatment goals in polycythemia vera: final results from the PROUD-PV/CONTINUATION-PV studies. HemaSphere. 2022;6(suppl 3):97-98. doi:10.1097/01.HS9.0000843676.80508.b5
10. Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial. Lancet Haematol. 2021;8(3):e175-e184. doi:10.1016/S2352-3026(20)30373-2

Leon-Ferre Explores Targeting of PIK3CA Alterations in ER+ Breast Cancer
July 24th 2024During a live Community Case Forum event in partnership with the Minnesota Society of Clinical Oncology, Roberto A. Leon-Ferre, MD, discussed drugs targeting PIK3CA alterations in patients with ER+ metastatic breast cancer.
Read More
Roundtable Roundup: Treatment for Metastatic pMMR Endometrial Cancer
July 23rd 2024In separate, live virtual events, Michael J. Birrer, MD, PhD, and Jubilee Brown, MD, surveyed participants on the treatment of a postmenopausal woman with stage IVA endometrial cancer after first-line chemotherapy.
Read More
George Explores Impact of Risk Status With Cabozantinib/Nivolumab in Advanced RCC
July 19th 2024During a Case-Based Roundtable® event, Daniel George, MD, discussed the results of the CheckMate 9ER trial across favorable, intermediate, and poor risk groups in patients with advanced renal cell carcinoma.
Read More
Depth of Response With Quadruplet Regimens Considered in Newly Diagnosed Multiple Myeloma
July 18th 2024During a Case-Based Roundtable® event, Timothy Schmidt, MD, and participants discussed treatment selection for a 54-year-old patient with transplant eligible R-ISS stage 2/R2-ISS stage 3 IgG-κ myeloma.
Read More
Rossetti Reviews Myelofibrosis Risk Stratification and Outcome Data for Pacritinib
July 17th 2024During a Case-Based Roundtable® event, James M. Rossetti, DO, discussed the role of risk scoring and stratification tools and treatment for a patient with declining hemoglobin and platelet counts due to primary myelofibrosis.
Read More
Phase 3 VERIFY Trial Investigates Rusfertide’s Potential in Polycythemia Vera
July 16th 2024In an interview, Aniket Bankar, MD, discussed the background, design, and goals of the phase 3 VERIFY trial of the hepcidin mimetic rusfertide for the treatment of patients with polycythemia vera.
Read More