GYNECOLOGIC CANCERS
Latest News
Latest Videos

More News

Bradley Monk, MD, gynecologic oncologist, University of Arizona Cancer Center Phoenix Branch, discusses trabectedin as a potential agent to treat ovarian cancer.

Most patients with ovarian cancer present with bulky and metastatic disease. Surgery and platinum-based chemotherapies are the mainstay of treatment for newly diagnosed disease, but recurrence/resistance are common.

Jyoti D. Patel, MD, discusses a phase II trial that looked at olaparib in combination with cediranib versus olaparib alone in recurrent platinum-sensitive ovarian cancer.
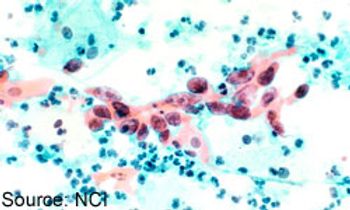
The cobas HPV Test was approved in 2014 by the FDA as a primary tool for cervical cancer screening in women ≥25 years of age, highlighting the need for updated guidelines to direct the use of approved screening methods in clinical practice.
Richard T. Penson, MD, Bradley J. Monk, MD, and Krishnansu S. Tewari, MD, weigh in on the changing landscape of the treatment of cervical cancer.

The poly (ADP-ribose) polymerase (PARP) inhibitor veliparib exhibits antitumor activity and is safe and tolerable on a continuous dosing schedule when used for the treatment of patients with BRCA-positive and BRCA-wild type tumors.
Patients with a rare but aggressive form of ovarian cancer had mutations in the chromatin regulator SMARCA4, suggesting a causative role of these mutations.

A study using next-generation sequencing showed that genetic testing limited to BRCA1/2 mutations would have missed 29% of mutations that carry hereditary risk of ovarian cancer.

A new study has recommended priority targets for immunotherapy in epithelial ovarian cancer (EOC) by profiling the expression of certain tumor-associated antigens (TAA), called MAGE cancer-testis antigens (CTA), in a large panel of tumor samples.

David Hyman, MD, medical oncologist, Memorial Sloan Kettering Cancer Center, discusses the results of a phase I study of MEDI3617 for advanced solid tumors.

The FDA has approved bevacizumab in combination with paclitaxel and cisplatin or paclitaxel and topotecan as a treatment for patients with persistent, recurrent, or metastatic cervical cancer, based on the improvement in OS in the phase III GOG 240 study.

Tanios Bekaii-Saab, MD, Section Chief, Gastrointestinal Oncology, associate professor, Internal Medicine, Pharmacology, The Ohio State University, discusses an analysis of two targeted drugs for the treatment of patients with colorectal cancer (CRC).

Cervical cancer screening based on identification of human papillomavirus (HPV) outperformed primary liquid cytology for detection of high-grade cervical intraepithelial neoplasia (CIN).

Bradley Monk, MD, gynecologic oncologist, University of Arizona Cancer Center Phoenix Branch, discusses the utility of bevacizumab for the treatment of cervical cancer.

The FDA has assigned a priority review designation to bevacizumab (Avastin) in combination with chemotherapy for patients with recurrent platinum-resistant ovarian cancer.

Amy P. Abernethy, MD, PhD, associate professor, School of Nursing, director, Duke Center for Learning Health Care, Duke University School of Medicine, discusses the results of a multisite randomized trial that examined continuing versus discontinuing statins in the setting of life-limiting illness.

The FDA has assigned a priority review designation to bevacizumab (Avastin) plus chemotherapy as a treatment for patients with persistent, recurrent, or metastatic cervical cancer.

The investigational CD19-targeted chimeric antigen receptor (CAR) therapy CTL019 has received a breakthrough therapy designation from the FDA as a potential treatment for pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

The FDA has granted a breakthrough therapy designation to blinatumomab for the treatment of adult patients with Philadelphia-negative relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL).

The FDA’s ODAC voted 11-2 against the accelerated approval of the PARP inhibitor olaparib as a maintenance therapy for women with platinum-sensitive relapsed ovarian cancer with germline BRCA mutations.

Major objective responses to treatment were demonstrated in one-fourth of patients with relapsed or refractory BRCA-mutant ovarian cancer with the investigational PARP inhibitor, veliparib, according to results from a phase II study.

Sean C. Dowdy, MD, professor, chair, Division of Gynecologic Surgery, co-leader, Women’s Cancer Program, Mayo Clinic, discusses bevacizumab and improvement of progression-free survival (PFS) for patients with the mesenchymal molecular subtype of ovarian cancer.

The FDA has approved the radioactive diagnostic imaging agent Lymphoseek injection to guide sentinel lymph node biopsy in patients with cancer of the head and neck.

Two patients with metastatic cervical cancer achieved durable complete responses that have so far lasted from 15 to 22 months through an adoptive T-cell therapy (ACT) targeting the human papillomavirus (HPV).

Combining the oral targeted agents olaparib and cediranib resulted in a near-doubling of median progression-free survival (PFS) among women with recurrent ovarian cancer.