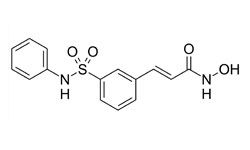
DLBCL
Latest News

A wide-ranging analysis of more than 5500 breast cancer tumors that combined genomic and protein expression testing has identified promising targets to explore for treating patients with poor prognoses, with particularly notable findings involving androgen receptor (AR) expression.
Latest Videos

CME Content
More News

Jennifer E. Amengual, MD, discusses the study she presented at the 2013 American Society of Hematology (ASH) Annual Meeting. The trial analyzed dual targeting with the HDAC inhibitor ACY-1215 and bortezomib in preclinical models of lymphoma.

Marcel R.M. van den Brink, MD, PhD, Head, Division of Hematologic Oncology, Alan N. Houghton Chair, Memorial Sloan-Kettering Cancer Center, highlights two studies that will be presented at the 2013 American Society of Hematology (ASH) Meeting.

The efficacy and safety of adding the experimental agent brentuximab vedotin (Adcetris) to standard chemotherapy to treat patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) is being evaluated in a randomized, open-label phase II trial.

Ari M. Melnick, MD, from Weill Cornell Medical College, comments on emerging therapies for the treatment of mantle cell lymphoma.
Several oral drugs are in development for both non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL). “There is different activity in different lymphoma types with different oral drugs and antibodies in development,†said Andrew D. Zelenetz, MD, PhD.

A Q&A with Andrew D. Zelenetz, MD, PhD, medical oncologist specializing in lymphoma at Memorial Sloan-Kettering Cancer Center, New York City.

Jennifer Woyach, MD, discusses the benefit of a combination therapy with ibrutinib for patients with chronic lymphocytic leukemia (CLL).

Daniel H. Fowler, MD, discusses the challenges of graph-versus-host-disease (GVHD) in patients with high-risk lymphoma.

Catherine Bollard, MBChB, MD, from the Baylor College of Medicine, discusses T cells and TGF-beta in Hodgkin's lymphoma and Non-Hodgkin lymphoma.

A dual-target PI3 kinase inhibitor demonstrated activity in several types of relapsed and refractory non-Hodgkin lymphoma.

At the 18th Annual Conference of the National Comprehensive Cancer Network (NCCN), experts presented the latest updates to the NCCN Clinical Practice Guidelines in Oncology.

Adding the BTK inhibitor ibrutinib to standard rituximab (R)-CHOP chemotherapy for non-Hodgkin lymphoma (NHL) resulted in an objective response rate of 100%.
The FDA has received a new drug application for ibrutinib as a therapy for previously treated CLL and previously treated MCL.
Patients with chronic lymphocytic leukemia (CLL) and patients with mantle cell lymphoma (MCL) showed high response rates to therapy with ibrutinib.

Julian Adams, PhD, President, Research and Development, Infinity Pharmaceuticals, describes a phase I trial of IPI-145, a potent inhibitor of PI3KEδ and PI3K-γ.

Lenalidomide has been approved to treat patients with mantle cell lymphoma who have relapsed or whose disease has progressed after two prior therapies including at least one prior treatment with bortezomib.

A phase III study of inotuzumab ozogamicin for patients with relapsed or refractory CD22+ aggressive non-Hodgkin lymphoma who are not candidates for high-dose chemotherapy was halted after a scheduled interim analysis.

Bruce D. Cheson, MD, Professor of Medicine, Head of Hematology, Director of Hematology Research, Georgetown Lombardi Comprehensive Cancer Center, describes the mechanism of action of antibody drug conjugates and their use.