HER2 Breast Cancer
Latest News
Latest Videos

More News

The adaptive I-SPY 2 trial has found that a neoadjuvant regimen of neratinib and standard chemotherapy is beneficial for high-risk patients with hormone receptor (HR)-negative, HER2-positive stage II/III breast cancer.

The combination of palbociclib and letrozole more than doubled PFS and showed a non–statistically significant 4.2-month improvement in OS for patients with ER-positive, HER2-negative metastatic breast cancer.

Sunil Verma, MD, MSEd, FRCPC, University of Toronto, Breast Medical Oncology, Sunnybrook Odette Cancer Centre, discusses advances in the treatment of patients with metastatic HER2-positive breast cancer.

The Journal of Targeted Therapies in Cancer discussed various issues regarding new and existing therapies in breast cancer with Richard Finn, MD.

Significant developments in research and treatment for HER2-positive breast cancer have emerged over the past year. Those advances include a growing number of studies measuring the effectiveness of dual agents.
Neoadjuvant and adjuvant therapies, administered before and after primary anticancer therapy, respectively, have been shown in numerous clinical trials to reduce recurrence and improve survival in patients with breast cancer.
Researchers have completed a comprehensive genomic analysis of cervical cancer in two patient populations. The study identified recurrent genetic mutations not previously found in cervical cancer, including one for which targeted agents have been approved in other cancers.

The I-SPY 2 trial, which is evaluating novel agents in the neoadjuvant treatment of breast cancer, may accelerate and influence the way that oncology drugs are tested and approved in the near future.
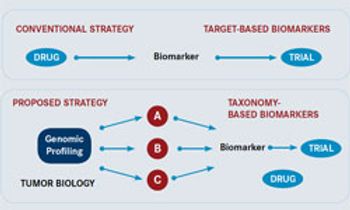
The development of predictive biomarkers is widely accepted as an important milestone in the move toward personalized, or precision, medicine and the ability to identify the right drug for the right patient. JTT interviewed Kopetz about biomarker models.

Findings reported at the 2013 San Antonio Breast Cancer Symposium showed that PIK3CA-mutated tumors in patients with HER2-positive (HER+) breast cancer (BC) are associated with a much lower rate of pathological complete response (pCR).

According to results from the large randomized BETH trial, bevacizumab did not improve invasive disease-free survival (IDFS) or overall survival (OS) when added to adjuvant therapy for HER2-positive breast cancer.

Aditya Bardia, MD, MPH, attending physician, Massachusetts General Hospital, Harvard Medical School, discusses the dual targeting of the HER2 receptor in patients with breast cancer.

Determination of HER2 status has become an important part of the workup of patients with advanced esophagogastric cancer, given recent data from a phase III trial (ToGA) indicating that trastuzumab, an anti-HER2 monoclonal antibody, prolongs survival in these patients.

Updated recommendations for HER2 testing in breast cancer have been released by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP).

Debu Tripathy, MD, discusses targeting HER2 in breast cancer.

The FDA’s Oncologic Drugs Advisory Committee voted 13-0 with one abstention in support of pertuzumab (Perjeta) in combination with trastuzumab and docetaxel for patients with HER2-positive breast cancer in the neoadjuvant setting.
Studies presented at the 2013 Breast Cancer Symposium investigated the efficacy and safety of various approaches to the treatment of HER2-positive breast cancer.

An analysis of the T-PAS expanded access study of T-DM1 in previously treated HER2+ metastatic breast cancer confirmed existing information on the safety of the combination, and observed significant clinical activity in a patient population that averaged seven prior therapies.

Denise A. Yardley, MD, Senior Investigator, Sarah Cannon Research Institute, discusses an expanded access study of T-DM1 in previously treated HER2-positive metastatic breast cancer.

Dual HER2-targeted neoadjuvant therapy for early breast cancer did not significantly improve the pCR rate compared with a single anti-HER2 agent plus chemotherapy.

Max S. Wicha, MD, was part of the first team to discover stem cells in breast cancer, and he is among the most highly cited investigators in the field of cancer stem cells.
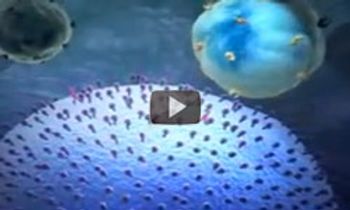
The mechanism of action of trastuzumab (Herceptin), a monoclonal antibody that is approved for the treatment of early-stage breast cancer that is HER2-positive.

Lisa A. Carey, MD, Professor of Medicine, University of North Carolina at Chapel Hill, discusses assays and intrinsic subtypes of breast cancer.

Martine Piccart, MD, PhD, director of medicine at the Jules Bordet Institute in Brussels, Belgium, discusses the changing view of anti-HER2 therapies for patients with breast cancer.