Colorectal Cancer
Latest News

Latest Videos

More News

Roberto Bordonaro, MD, chair, medical oncology, Garibaldi Hospital, Catania, Italy, discusses first-line treatment options for advanced colorectal cancer (CRC).

Marc Peeters, MD, PhD, department of oncology, Antwerp University Hospital, Antwerpen, Belgium, comments on the future of the treatment of patients with colorectal cancer.

The FDA’s Molecular and Clinical Genetics advisory committee has unanimously supported the safety, efficacy, and positive risk-benefit profile of the noninvasive stool-based DNA test Cologuard in a 10-0 vote.
The blood-based colorectal cancer (CRC) screening test Epi proColon passed the scrutiny of the FDA’s Molecular and Clinical Genetics advisory panel in a close 5-4 vote with 1 abstention in support of the claim that the test’s benefits outweigh its risks.

Marc Peeters, MD, PhD, department of oncology, Antwerp University Hospital, Antwerpen, Belgium, discusses an analysis of RAS mutations in the phase III study 20050181, which compared panitumumab plus FOLFIRI versus FOLFIRI for second-line treatment of metastatic colorectal cancer.

RAS mutations beyond KRAS exon 2 are negative predictive biomarkers for panitumumab in mCRC, according to an analysis of phase III, second-line data, which are consistent with previously reported data in first-line mCRC.

Findings from the NSABP R-04 trial demonstrated that neoadjuvant capecitabine combined with radiation therapy had outcomes similar to previously established standards of care for stage II or III rectal cancer.

Roberto Bordonaro, MD, chair, medical oncology, Garibaldi Hospital, Catania, Italy, discusses the treatment of patients with advanced colorectal cancer.
Studies presented at the 2014 GI Cancers Symposium emphasized the need for full RAS mutational analyses in the management of mCRC prior to initiating treatment with anti-EGFR monoclonal antibodies

Charles S. Fuchs, MD, MPH, director, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, on the use of aspirin and targeting COX-2 in colorectal cancer.

RAS mutations beyond KRAS exon 2 are negative predictive biomarkers for the EGFR-inhibitor panitumumab in metastatic colorectal cancer, according to an analysis of phase III, second-line data, which are consistent with previously reported data in first-line mCRC.

According to findings from a four-arm phase III NSABP R-04 trial, single-agent neoadjuvant capecitabine combined with radiation therapy demonstrated similar outcomes as previously established standards of care for patients with stage II or stage III rectal cancer.
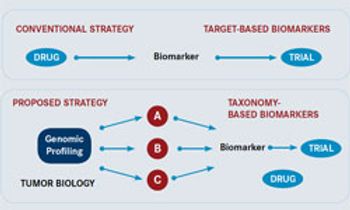
The development of predictive biomarkers is widely accepted as an important milestone in the move toward personalized, or precision, medicine and the ability to identify the right drug for the right patient. JTT interviewed Kopetz about biomarker models.

In September 2012, the FDA approved regorafenib for treatment of patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild-type, with an anti-EGFR therapy.

Recent biomarker analyses of studies involving the anti-EGFR antibody panitumumab combined with chemotherapy for treatment of mCRC have advanced clinicians’ ability to prospectively identify patients who are, more or less, likely to respond to this agent.

A post-hoc analysis of the VELOUR trial demonstrated additional improvements in both overall survival (OS) and progression-free survival (PFS) in favor of aflibercept plus FOLFIRI in patients with mCRC.

Charles S. Fuchs, MD, MPH, director, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, discusses targeting KRAS in colorectal cancer.

Julie R. Brahmer, MD, from Johns Hopkins University School of Medicine, Sidney Kimmel Comprehensive Cancer Center, discusses the outlook for immunotherapies in cancer care.

S. Gail Eckhardt, MD, discusses the role of regorafenib in colorectal cancer.

S. Gail Eckhardt, MD, discusses the role of angiogenesis inhibitors for the treatment of metastatic colorectal cancer (mCRC), following the approval of aflibercept.

Regorafenib is a recently approved oral, multikinase inhibitor for refractory colorectal cancer. Although structurally similar to sorafenib, it more potently inhibits a broader spectrum of critical growth receptor pathways, including those regulating angiogenesis and aberrant cellular proliferation.

Over the past two decades, there has been a shift away from indiscriminate cell-killing by anticancer agents toward the development of more specific drugs that target key aspects of cancer cell biology.

S. Gail Eckhardt, MD, Professor and Head of the Division of Medical Oncology at the University of Colorado Denver and Health Sciences Center, discusses the wnt pathway and its role in colorectal cancer.

Paul A. Bunn, Jr, MD, from the University of Colorado, discusses afatinib for patients with activating epidermal growth factor receptor mutation.

Carol Aghajanian, MD, from the Memorial Sloan-Kettering Cancer Center, discusses the difficulties with a gold standard clinical trial endpoint in ovarian cancer.