
Based on data from the phase III RAINBOW trial, the FDA has approved ramucirumab (Cyramza) in combination with paclitaxel as a treatment for patients with previously treated advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma.

Your AI-Trained Oncology Knowledge Connection!



Based on data from the phase III RAINBOW trial, the FDA has approved ramucirumab (Cyramza) in combination with paclitaxel as a treatment for patients with previously treated advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma.

William D. Tap, MD, discusses the potential to combine KIT and CTLA-4 blockade in patients with refractory GIST and other advanced sarcomas.

A head-to-head comparison of cetuximab and bevacizumab showed equivalence for chemotherapy plus either agent in terms of OS, PFS, and response rates for patients with certain previously untreated mCRC.

Tanios Bekaii-Saab, MD, Section Chief, Gastrointestinal Oncology, associate professor, Internal Medicine, Pharmacology, The Ohio State University, discusses an analysis of two targeted drugs for the treatment of patients with colorectal cancer (CRC).

Last week, the US Food and Drug Administration (FDA) announced two steps it will take to ensure the safety and utility of certain diagnostic tests.

The investigational CD19-targeted chimeric antigen receptor (CAR) therapy CTL019 has received a breakthrough therapy designation from the FDA as a potential treatment for pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

Raoul S. Concepcion, MD, FACS, Urology Associates P.C., discusses the potential for the 4Kscore test as a predictor of high-grade prostate cancer.

The FDA has approved the radioactive diagnostic imaging agent Lymphoseek injection to guide sentinel lymph node biopsy in patients with cancer of the head and neck.

A cell-cycle gene array test demonstrated independent value for predicting metastatic progression after surgery for organ-confined renal cell carcinoma (RCC) of clear cell histology.

Ghassan K. Abou-Alfa, MD, Gastrointestinal Oncology Service, Memorial Sloan Kettering Cancer Center, discusses a retrospective study to determine the prognostic value of C-reactive protein (CRP) levels in patients with hepatocellular carcinoma (HCC) who are undergoing treatment with sorafenib.
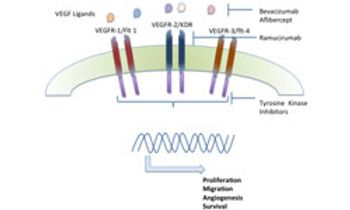
Advanced gastric cancer and gastroesophageal junction cancers are highly aggressive cancers with poor prognosis and limited treatment options. The VEGF pathway has been one of the key signaling pathways under investigation for these cancers.

Marcel R.M. van den Brink, MD, PhD, from Memorial Sloan Kettering Cancer Center, describes a novel CLIA-certified next-generation sequencing-based assay for hematologic malignancies.
The FDA has approved ramucirumab as a treatment for patients with unresectable gastric cancer or GEJ adenocarcinoma following fluoropyrimidine- or platinum-containing therapy, based on a significant extension in overall survival (OS).

Ghassan K. Abou-Alfa, MD, Gastrointestinal Oncology Service, Memorial Sloan-Kettering Cancer Center, discusses the phase II study of a novel transforming growth factor-beta receptor I (TGF-β1) kinase inhibitor, LY2157299 monohydrate, in patients with advanced hepatocellular carcinoma (HCC), which was presented at the 2014 Gastrointestinal Cancers Symposium

Screening for prostate-specific antigen (PSA) significantly cuts the death rate from prostate cancer, but at the same time, America’s medical community should work harder to avoid the screen’s potential pitfalls.

A wide-ranging analysis of more than 5500 breast cancer tumors that combined genomic and protein expression testing has identified promising targets to explore for treating patients with poor prognoses, with particularly notable findings involving androgen receptor (AR) expression.

Sukumar Nagendran, MD, vice president, medical affairs, Quest Diagnostics, describes BRCAvantage, a test to detect BRCA1 and BRCA2 genes.

Vicki Keedy, MD, Assistant Professor of Medicine, Clinical Director, Sarcoma Program, Vanderbilt-Ingram Cancer Center, discusses novel agents that are currently under investigation for the treatment of gastrointestinal stromal tumors

Julie R. Brahmer, MD, from Johns Hopkins University School of Medicine, Sidney Kimmel Comprehensive Cancer Center, discusses the outlook for immunotherapies in cancer care.

Determination of HER2 status has become an important part of the workup of patients with advanced esophagogastric cancer, given recent data from a phase III trial (ToGA) indicating that trastuzumab, an anti-HER2 monoclonal antibody, prolongs survival in these patients.

Meredith C. Henderson, PhD, Head, R&D, Provista Diagnostics, describes the dtectDx Breast test.

Over the past two decades, there has been a shift away from indiscriminate cell-killing by anticancer agents toward the development of more specific drugs that target key aspects of cancer cell biology.

Paul A. Bunn, Jr, MD, from the University of Colorado, discusses afatinib for patients with activating epidermal growth factor receptor mutation.

Carol Aghajanian, MD, from the Memorial Sloan-Kettering Cancer Center, discusses the difficulties with a gold standard clinical trial endpoint in ovarian cancer.

Renier J. Brentjens, MD, PhD, from Memorial Sloan-Kettering Cancer Center, discusses the potential efficacy of CAR-modified T cells for the treatment of solid tumors.