HEMATOLOGY
Latest News
Latest Videos

More News

Timothy S. Pardee, MD, from Wake Forest University Baptist Medical Center, discusses the mechanism and utility of the pyruvate dehydrogenase complex inhibitor CPI-613 for the treatment of patients with advanced hematologic malignancies.

Jasmine Zain, MD, discusses how her institution is working on improving the outcome of patients with T cell lymphoma that undergo transplants.

Jae Park, MD, attending physician, Leukemia Service, Memorial Sloan-Kettering Cancer Center, describes the design of a trial analyzing vemurafenib for the treatment of patients with hairy cell leukemia.

Miguel-Angel Perales, MD, from Memorial Sloan-Kettering Cancer Center, discusses the challenges regarding access to bone marrow transplant donors.

Mark J. Levis, MD, PhD, discusses the ideal way to use quizartinib to treat a patient with an FLT3-ITD mutation in acute myeloid leukemia (AML).
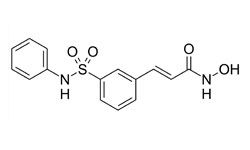
Several oral drugs are in development for both non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL). “There is different activity in different lymphoma types with different oral drugs and antibodies in development,†said Andrew D. Zelenetz, MD, PhD.

Renier J. Brentjens, MD, PhD, associate professor, chief, Cellular Therapeutics Center, Memorial Sloan-Kettering Cancer Center, discusses the differences between the CAR-modified T cells of three institutions.

The FDA approval of two anti-CD20 antibodies—ofatumumab (Arzerra) and, just recently, obinotuzumab (Gazyva)—has greatly advanced the outlook for managing chronic lymphocytic leukemia.

Idelalisib is a potent and highly selective PI3K inhibitor that promotes apoptosis in primary cells from patients with different B-cell malignancies. Idelalisib has been shown to affect microenvironmental signaling and cell survival both in vitro and in vivo.

Richard R. Furman, MD, Richard A. Stratton Assistant Professor in Hematology and Oncology, Weill Cornell Medical College, gives an overview of the efficacy and safety of idelalisib and rituximab for previously treated CLL.

Ayalew Tefferi, MD, hematologist, Mayo Clinic, Rochester, Minnesota, discusses the outlook for JAK inhibitors for patients with myelofibrosis.

A combination of rituximab (Rituxan) and the PI3K-delta inhibitor idelalisib was associated with a >70% improvement in overall survival (OS) in patients with high-risk relapsed/refractory chronic lymphocytic leukemia (CLL).

At the 55th Annual Meeting of the American Society of Hematology (ASH), several trials of ibrutinib both alone and in combination with currently used therapies for patients with chronic lymphocytic leukemia (CLL) were presented.

Results of a phase II trial showed that when treated with a reduced dose of the oral FLT3 receptor tyrosine kinase inhibitor quizartinib, nearly half of patients with relapsed/refractory acute myelogenous leukemia (AML) had complete remissions.

Brentuximab vedotin, an anti-CD30 monoclonal antibody, has demonstrated antitumor activity in the setting of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and has generated responses across a broad range of CD30 expression, including low or undetectable CD30 expression. Data from an ongoing phase II study were presented by Nancy Bartlett, MD, at the 55th annual meeting of the American Society of Hematology (ASH).

Jennifer E. Amengual, MD, discusses the study she presented at the 2013 American Society of Hematology (ASH) Annual Meeting. The trial analyzed dual targeting with the HDAC inhibitor ACY-1215 and bortezomib in preclinical models of lymphoma.

In patients with previously untreated chronic lymphocytic leukemia (CLL) who are considered inappropriate for fludarabine, the addition of ofatumumab to chlorambucil improves clinical outcomes and is tolerable irrespective of patient age or fitness.

According to preliminary results of a phase I clinical trial, nearly half of patients with relapsed or refractory CLL attained objective responses when treated with IPI-145, an oral inhibitor of PI3K-delta and -gamma.

An orally bioavailable selective inhibitor of the Bcl-2 protein induced remissions in patients with relapsed/refractory CLL and SLL.

A randomized trial showed that patients with CLL and major comorbidities had significantly better outcomes when treated with obinutuzumab, an anti-CD20 monoclonal antibody, instead of rituximab.

Jennifer Brown, MD, PhD, discusses final stage 2 results of the CLL11 trial at the 2013 American Society of Hematology (ASH) Meeting.

Results of a small clinical study showed that in patients with posttransplant relapsed B-cell malignancies, treatment with engineered donor T cells led to substantial tumor regression.

Mark J. Levis, MD, PhD, discusses the difficulty of treating a patient with a FLT3-ITD mutation in acute myeloid leukemia.

Marcel R.M. van den Brink, MD, PhD, Head, Division of Hematologic Oncology, Alan N. Houghton Chair, Memorial Sloan-Kettering Cancer Center, highlights two studies that will be presented at the 2013 American Society of Hematology (ASH) Meeting.

Jae Park, MD, attending physician, Leukemia Service, Memorial Sloan-Kettering Cancer Center, comments on the treatment of patients with hairy cell leukemia.