
Lawrence Fong, MD, discusses the systemic antitumor effect and clinical response in a phase II trial of intratumoral electroporation of plasmid interleukin-12 (IL-12) in patients with advanced melanoma.

Your AI-Trained Oncology Knowledge Connection!


Lawrence Fong, MD, discusses the systemic antitumor effect and clinical response in a phase II trial of intratumoral electroporation of plasmid interleukin-12 (IL-12) in patients with advanced melanoma.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the potential to partner T-VEC with a targeted agent for the treatment of patients with melanoma.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses the potential for immunotherapy combinations for the treatment of melanoma.

Last week, the FDA approved pembrolizumab (Keytruda) for the treatment of patients with advanced or unresectable melanoma following progression on prior therapies.

Since the FDA approval of ipilimumab, there has been considerable interest in the potential for other immune system checkpoints, such as PD-L1 and its receptor, PD-1, to serve as therapeutic targets in metastatic melanoma.

Bristol-Myers Squibb has filed a lawsuit over Merck’s newly approved immunotherapy drug pembrolizumab, contending that the much-heralded PD-1 inhibitor will infringe upon patents that Bristol-Myers holds on the groundbreaking technology.

A novel HER2-derived peptide (GP2) vaccine designed to stimulate CD8+ cytotoxic T-lymphocytes reduced the rate of breast cancer recurrence and proved safe and well tolerated in an adjuvant setting.

The FDA has approved pembrolizumab (Keytruda) as a treatment for patients with advanced or unresectable melanoma following progression on prior therapies.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the rationale behind a phase Ib study to evaluate the efficacy and safety of T-VEC and ipilimumab in previously untreated, unresected stage IIIB-IV melanoma
Advaxis and Merck have entered into a clinical trial collaboration agreement to evaluate the combination of two novel immunotherapies for the treatment of patients with metastatic castration-resistant prostate cancer.

Ezra Cohen, MD, professor of medicine, UC San Diego Moores Cancer Center, discusses immunotherapy in head and neck cancer.

A multidisciplinary approach has become increasingly important in the treatment of HNC in order to provide effective, timely, and evidence-based management of these complex and heterogeneous tumors.
William B. Coley, MD, now known as the Father of Immunotherapy, first attempted to harness the immune system for treating cancer in the late 19th century.

Biomarkers are needed to fulfill several important roles in immuno-oncology, both before and after treatment.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses a phase I trial that examined the combination of nivolumab and ipilimumab for the treatment of advanced melanoma. The data presented at the 2014 ASCO Annual Meeting was an updated survival and clinical activity analysis in initially enrolled cohorts and activity by BRAF mutation status.
Using the patient’s immune system to target and destroy cancer cells has long been a sought-after goal in oncology.

Gregory A. Daniels, MD, PhD, discusses opportunities to combine immunotherapies with both targeted therapies and other immunotherapies.

Jedd D. Wolchok, MD, PhD, medical oncologist, Memorial Sloan Kettering Cancer Center, discusses the management of side effects associated with immunotherapies.

Advances in our understanding of critical immunomodulatory checkpoints, such as the cytotoxic T-lymphocyte antigen 4 (CTLA-4), have led to some recent notable successes in the immunotherapy of cancer.

James P. Allison, PhD, director, immunotherapy platform, The University of Texas MD Anderson Cancer Center, discusses the future of immune checkpoint strategies.

A new study has recommended priority targets for immunotherapy in epithelial ovarian cancer (EOC) by profiling the expression of certain tumor-associated antigens (TAA), called MAGE cancer-testis antigens (CTA), in a large panel of tumor samples.

The development of new immunotherapies for cancer treatment generated significant interest at the 2014 ASCO Annual Meeting, particularly checkpoint inhibitors targeting the PD-1 receptor and its ligand, PD-L1.
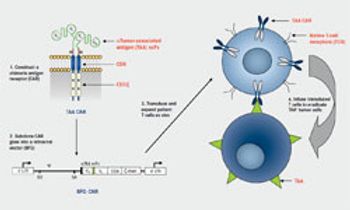
For nearly 2 decades, researchers have been exploring B-cell–specific antigens in hopes of developing a new anticancer target that could surpass the success of CD20-targeting agents.

Despite standard chemotherapy and the availability of targeted therapies such as bevacizumab, cetuximab, and tyrosine kinase inhibitors (TKIs) such as erlotinib, afatinib, and crizotinib, survival rates are far from optimal for patients with NSCLC.

The concept of cancer immunotherapy— using the patient’s immune system to target and eradicate cancer—has evolved over more than a century of research.