IMMUNOTHERAPY
Latest News
Latest Videos

More News

Pembrolizumab (Keytruda) achieved an overall response rate (ORR) of 45.2% among a cohort of patients with high PD-L1-expressing non–small cell lung cancer (NSCLC) in the phase I KEYNOTE-001 trial.
Pembrolizumab (Keytruda) yielded significantly better outcomes compared with ipilimumab (Yervoy) in a randomized phase III trial of patients with advanced melanoma.

IMCgp100, an anti-CD3 antibody fragment fused to a gp100-specific T cell receptor, yielded long-lasting responses in patients with advanced melanoma.

Kevin B. Kim, MD, medical oncology, California Pacific Medical Center, discusses the outlook for T-VEC for the treatment of patients with melanoma.

Novel combinations and immunotherapies have significantly expanded treatment options for myeloma. Numerous studies, including the ASPIRE and ELOQUENT-2 trials, have shown positive results for triple drug combinations.

A 2:1 open-label phase II trial of the FANG vaccine achieved a marked delay in time to progression, in all 14 of 21 patients with stage III/IV ovarian cancer who participated. The other 7 patients did not receive the vaccine.

A trial comparing frontline pembrolizumab with ipilimumab for the treatment of advanced melanoma has met its progression-free survival (PFS) and overall survival (OS) endpoints and will be stopped early.

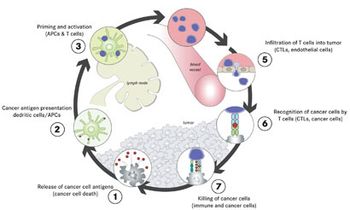
Immunotherapy vaccine approaches that involve harvesting a patient’s dendritic cells and priming them to recognize a tumor-specific antigen before injecting them back into the patient to activate a T-cell response against the tumor have achieved encouraging but limited success.

Heather Wakelee, MD, associate professor of medicine, oncology division, Stanford University, discusses the rationale for immunotherapy in non-small cell lung cancer (NSCLC).
Cancer proliferates when a rogue, transformed cell wins a sophisticated hide-and-seek game against the immune system. Immunotherapy activates the patient’s immune system to recognize and fight the tumor cells.

Immunotherapy is a rapidly expanding approach to the treatment of melanoma, employing a number of strategies evident in the pipeline for immunotherapeutics.
Melanoma is the most deadly skin cancer. According to data collected between 2004 and 2010, only 16% of Americans with metastatic melanoma, including people of all ages and races, and both genders, survive for 5 years or more after diagnosis.
Following recent approvals by the US Food and Drug Administration (FDA) of pembrolizumab and nivolumab, numerous programs to develop and expand use of immunotherapies have ensued.

Jason J. Luke, MD, assistant professor of medicine, The University of Chicago, discusses PD-1 inhibitors for the treatment of patients with melanoma.

Mario Sznol, MD, professor of medicine, Yale Cancer Center, discusses the role of high-dose IL-2 for the treatment of renal cell carcinoma (RCC).

Omid Hamid, MD, discusses sequencing and combining targeted therapies in the treatment of melanoma.
An immune checkpoint inhibitor combined with active immunotherapy has a potential positive effect on overall survival (OS) in the treatment of metastatic castration-resistant prostate cancer (mCRPC).
In an analysis of adverse events following treatment of patients with advanced melanoma with ipilimumab and nivolumab, combination therapy was associated with a 22% incidence of either thyroiditis or hypothyroidism and a 9% incidence of hypophysitis.

MPDL3280A, an investigational antibody that targets programmed death-ligand 1 (PD-L1), in combination with bevacizumab had strong antitumor activity and induced responses in 4 of 10 patients with mRCC.

The FDA has approved nivolumab (Opdivo) for the treatment of patients with advanced non-small cell lung cancer (NSCLC). The approval comes 3 months ahead of the FDA’s scheduled decision date.

The FDA has accepted a supplemental Biologics License Application (sBLA) for ipilimumab (Yervoy) as an adjuvant treatment of patients with stage III melanoma at high risk of recurrence following complete resection.
Nivolumab (Opdivo) has been granted a priority review for use in patients with previously treated, advanced, squamous non–small cell lung cancer (NSCLC).

Jae Park, MD, assistant attending physician, Memorial Sloan Kettering Cancer Center, discusses toxicities associated with CAR-modified T cell therapy.
An orphan drug designation has been granted to Reolysin (wild-type reovirus) for the treatment of patients with ovarian cancer.

The FDA has scheduled a hearing to discuss the biologics license application (BLA) for the immunotherapy talimogene laherparepvec (T-VEC) as a treatment for patients with metastatic melanoma.