IMMUNOTHERAPY
Latest News
Latest Videos
More News

Kei Muro, MD, discusses phase II and III trials which examine pembrolizumab as a treatment for patients with advanced gastric cancer.

The anti–PD-L1 agent MPDL3280A has received a breakthrough therapy designation from the FDA as a potential treatment for patients with PD-L1–positive NSCLC who have progressed on platinum-based chemotherapy and a EGFR or ALK inhibitor.
Metastatic disease accounts for the vast majority of cancer-related deaths. Ensuring a definitive diagnosis and the most effective treatment in a timely fashion is essential for extending life expectancy.

Pembrolizumab showed promising antitumor activity and a manageable toxicity profile in patients with metastatic gastric cancer, according to updated findings from the KEYNOTE-012 study.
The start of 2015 brought news from Novartis that it had signed an agreement with Intellia Therapeutics and Caribou Biosciences to license its proprietary CRISPR/Cas9 gene editing platform to develop novel treatments for chronic genetic-based diseases.

Nivolumab (Opdivo) improved survival compared with docetaxel in patients with pretreated squamous cell non–small cell lung cancer (NSCLC) in the phase III CheckMate-017 trial, according to Bristol-Myers Squibb (BMS), which manufactures the drug.

One of the leading non-clinical attributes that affect how oncologists decide which therapeutic agent to prescribe over another is patient affordability.

Amgen and Kite Pharma have announced that they will collaborate on the development of novel CAR T-cell immunotherapies, with Amgen providing cancer targets and Kite offering its engineered autologous cell therapy platform.

The FDA approved nivolumab for patients with advanced melanoma in December 2014, joining 6 other melanoma treatments approved in the past 3 years, including monoclonal antibodies pembrolizumab and ipilimumab.

The FDA has approved nivolumab (Opdivo) for patients with unresectable or metastatic melanoma following treatment with ipilimumab or a BRAF inhibitor, based on data from the phase III CheckMate-037 trial.

Edith A. Perez, MD, the deputy director at large for the Mayo Clinic Cancer Center, comments on immune checkpoint inhibition for the treatment of breast cancer.

Sara Hurvitz, MD, medical oncologist, UCLA Medical Center, discusses the results of a study looking at pembrolizumab (Keytruda) in patients with triple-negative breast cancer (TNBC).

Jae Park, MD, assistant attending physician, Memorial Sloan Kettering Cancer Center, discusses CAR T-cell therapy for the treatment of acute lymphoblastic leukemia.

The anti-CD19 chimeric antigen receptor (CAR)-modified T-cell therapy CTL019 demonstrated a 92% complete response (CR) rate in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

Most patients with classical Hodgkin lymphoma (cHL), having previously failed three or more therapies, responded to the immunotherapy nivolumab in a small phase I trial.

Treatment with the PD-1 inhibitor pembrolizumab (Keytruda) elicited responses in 66% of patients with classical Hodgkin lymphoma (cHL).

Philippe Armand, MD, PhD, senior physician, Dana-Farber Cancer Institute, discusses the utility of PD-1 inhibitors for hematologic malignancies.

Pfizer and Merck KGaA will collaborate on the development of the PD-L1 inhibitor MSB0010718C as a potential treatment for multiple types of cancer, according to the companies.

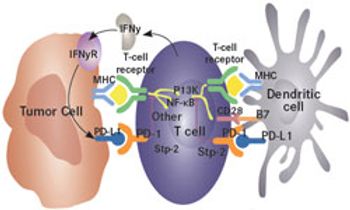
The programmed death receptor-1 (PD-1) ligand, PD-L1, has become a viable target for immunotherapy in cancer, with multiple antibodies now in development.

Clinicians will soon face challenging decisions on which immunotherapies to prescribe their patients, and in what sequence or combination.

Multiple trials are ongoing in NSCLC with immunotherapy agents. As with most cancers, however, there is also a need to identify which types of patients with NSCLC might benefit the most from these new therapies.

Vassiliki Papadimitrakopoulou, MD, discusses adverse events associated with immunotherapies for the treatment of lung cancer.

In malignancies with limited response rates to existing radiation and/or chemotherapy, immunotherapy is being investigated as a potential adjunct, with promising results, particularly in the treatment of malignant mesothelioma.

Barbara Burtness, MD, discusses potential immunotherapy agents that may assist in the treatment of head and neck cancers.
It is estimated that 1 in 63 individuals in the United States will develop renal cell carcinoma (RCC), making it among the most common cancers in the country.