IMMUNOTHERAPY
Latest News
Latest Videos

More News

This feature covers the "Checkpoint Inhibitors Under Development For Other Cancers" and "Summary" section of the current Evolving Paradigms in Immuno-Oncology issue.

This feature covers the "Treatment Strategies and Clinical Evidence for Checkpoint Inhibiton" section of the current Evolving Paradigms in Immuno-Oncology issue.

This feature covers the "A New Paradigm for Evaluation of Tumor Response and Adverse Events" section of the current Evolving Paradigms in Immuno-Oncology issue.

This feature covers the "Introduction" and "Overview of Mechanisms" sections of the current Evolving Paradigms in Immuno-Oncology issue.

The PD-1 inhibitor pembrolizumab in combination with an established multiple myeloma regimen showed responses in 76% of a cohort of 17 patients with relapsed/refractory disease in data one of several clinical trials presented at the 2015 ASH Annual Meeting.
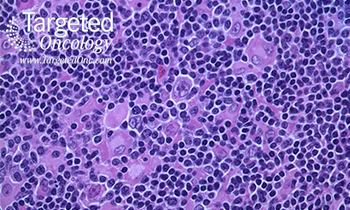
In this review, our authors discuss the mechanism of action and clinical development of various checkpoint inhibitors in lymphoma.

Ola Landgren, MD, PhD, on the progression treatments from combinations toward three-drug treatments and the use of monoclonal antibodies. Landgen adds that while he does have faith that monoclonal antibodies will change the treatment landscape in oncology, he says they could very well be combined with old and new drugs.

Rindopepimut is the first immunotherapy to have ever shown a survival benefit in brain cancers, specifically glioblastoma, said David Reardon, MD.

Danae Delivanis, MD, endocrinologist, Mayo Clinic, on the use of pembrolizumab and nivolumab in the treatment of metastatic melanoma.

The FDA has approved nivolumab (Opdivo) as a treatment for patients with metastatic renal cell carcinoma following prior treatment with an anti-angiogenic therapy.

Amy Heimberger, MD, professor in the Department of Neurosurgery, University of Texas MD Anderson Cancer Center, talks about immunotheraputics in the context of glioblastoma (GBM).

A quarter of patients with relapsed glioblastoma multiforme (GBM)-treated with the vaccine rindopepimut (Rintega) plus bevacizumab remained alive at 2 years.

Phase II findings have shown a substantial improvement in overall survival with the dendritic vaccine ICT-107 in a subgroup of patients with HLA-A2+ newly diagnosed glioblastoma multiforme (GBM), warranting further exploration in a phase III study.

Dr. Suresh Ramalingam on sequencing immune checkpoint inhibitors with chemotherapy in NSCLC treatment. He adds that a new class of agents can be given in conjunction with immune checkpoint inhibitors, such as IDO inhibitors.

The treatment paradigm for renal cell carcinoma (RCC) could undergo major changes in the future due to several novel therapies in the works for regulatory approval and checkpoint inhibitors, vaccines, recombinant T-cell receptors, and bi-specific T-cell engagers.

An FDA panel voted against approval of the immunotherapy MCNA as a treatment for patients with high-risk non-muscle invasive bladder cancer (NMIBC) following first-line bacillus Calmette-Guerin (BCG) therapy.

Bladder cancer may soon catch up to other areas of oncology in terms of an influx of immunotherapies, according to Thomas Powles, MD.

Immunotherapy is fast becoming the new standard of care for non-small-cell lung cancer alongside developing research in the field.

Chandra P. Belani, MD, deputy director, Penn State Hershey Cancer Institute, Miriam Beckner Distinguished Professor of Medicine, Penn State Hershey College of Medicine, talks about how immunotherapies targeting PD-L1 and PD-1 are showing activity in lung cancer.

While immune checkpoint inhibitors initially showed promise in patients with ovarian cancer, results still need to be validated by larger randomized trials.

Paul Walfish, MD, professor emeritus at the University of Toronto School of Medicine, senior consult Mount Sinai Hospital discusses programmed death ligand 1 (PD-1) expression in aggressive metastatic papillary thyroid cancer.

Danae Delivanis, MD, Division of Endocrinology, Diabetes, Metabolism, and Nutrition at Mayo Clinic, discusses pembrolizumab-induced thyroiditis in patients with cancer.

No established regimens exist for the treatment of patients with advanced hepatocellular carcinoma after failure of first-line treatment with the multikinase inhibitor sorafenib. Outcomes in the setting of sorafenib resistance or intolerance are poor, with a median expected overall survival for the placebo arms of second-line trials in the range of 7 to 8 months.
In recent years, knowledge of hepatocellular carcinoma (HCC)-specific tumor-associated antigens and the development of immune checkpoint blockade therapy has offered a positive outlook for patients with HCC.

Three months ahead of schedule, nivolumab (Opdivo) has obtained FDA approval for patients with nonsquamous non-small cell lung cancer who progressed on or following platinum-based chemotherapy.