MULTIPLE MYELOMA
Latest News
Latest Videos

More News

Updated findings from the CARTITUDE-1 trial presented at the 2021 ASH Annual Meeting and Exposition show that the use of a CAR T-cell therapy resulted in durable responses that lasted at nearly 2 years of follow-up across most subgroups with relapsed/refractory multiple myeloma.

Venetoclax dosed at either 400 mg or 800 mg in combination with daratumumab and dexamethasone showed promising preliminary results in a phase 1/2 study.

Having knowledge that their multiple myeloma was incurable was associated with poor quality of life outcomes in patients.

The XVd regimen appears effective at prolonging progression-free survival with decreased toxicity when administered at lower doses of selinexor or the standard dose.

Updated results from CARTITUDE-1 reveal deep and durable response to ciltacabtagene autoleucel treatment in patients with multiple myeloma.

An early and deep response was seen with a single infusion of cilta-cel in patients with multiple myeloma who experienced early clinical relapse.

A more durable and deep responses was produced with the recommended phase 2 dose of selinexor plus pomalidomide and dexamethasone compared with the lesser selinexor dose for relapsed or refractory multiple myeloma.

In patients with relapsed/refractory multiple myeloma with t(11;14), Selinexor plus venetoclax induced decreases in cyclin D1, XPO1, and MCL-1.

Compared with standard of care, ciltacabtagene autoleucel produces better responses in the setting of heavily pretreated multiple myeloma.
Results from the phase 2 IFM 2018-01 trial show positive safety and efficacy for the combination of daratumumab with ixazomib, lenalidomide, and dexamethasone.

The XVd regimen demonstrated comparable efficacy and safety in patients with multiple myeloma who had high-risk or standard-risk cytogenetic features.

The use of isatuximab with the RVd regimen in the induction phase may improve minimal residual disease negativity rates in patients with multiple myeloma, phase 3 results show.

No overlapping toxicities and a tolerable safety profile were seen with the addition of talquetamab to daratumumab in a population of patients with relapsed/refractory multiple myeloma.

Higher sustained MRD-negativity rates were observed among patients with transplant-eligible newly diagnosed multiple myeloma receiving D-VTd over VTd alone

In frail and elderly patients with multiple myeloma who are treated in the relapsed setting, a phase 2 trial shows promise of the daratumumab/ixazomib combination when given without dexamethasone.

Compared to those who waited and received second line daratumumab-based regimens for transplant-ineligible newly diagnosed multiple myeloma, patients receiving daratumumab, lenalidomide, and dexamethasone in the first line saw better overall survival.

A combination of ixazomib, daratumumab, and low-dose dexamethasone elicited an objective response rate (ORR) of 71%, in NDMM.

The approval is based on the phase 2 PLEAIDES study, which found the agent had an ORR of 84.8%, with 82.5% of patients still responding after 9 months.

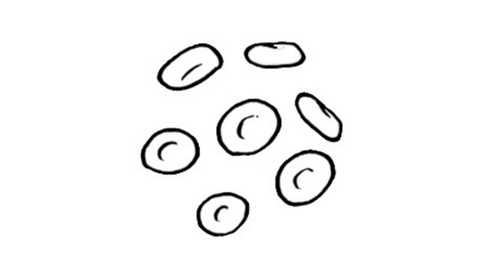
A 51-year-old man presented with worsening fatigue on exertion and pallor with an ECOG performance score of 2 and was diagnoses with stage II standard-risk multiple myeloma.

In an interview with Targeted Oncology, Ruben Niesvizky, MD, discussed current efficacy outcomes in multiple myeloma and how the introduction of the car-BIRD regimen may improve upon them.

GC012F, a duel target BCMA/CD19 CAR T-cell therapy was granted an orphan drug designation after durable responses were seen in a phase 1 study of the agent.

A 51-year-old man presented with worsening fatigue on exertion and pallor with an ECOG performance score of 1. He eventually received a diagnosis of stage II standard-risk multiple myeloma after testing and examination.

A 51-year-old man presented with pallor and worsening fatigue on exertion and was later diagnoses with multiple myeloma.

Shaji Kumar, MD, discusses how risk assessments and new drug combinations could influence treatment of patients with newly diagnosed multiple myeloma.

A 51-year-old man presented with worsening fatigue on exertion and pallor, with an ECOG performance score of 1. He eventually received a diagnosis of stage II standard-risk multiple myeloma after testing and examination.