
Ovarian Cancer
Latest News
Latest Videos

CME Content
More News

Jeffrey Infante, MD, director, Drug Development Program, Sarah Cannon Research Institute, discusses a phase Ib, open-label expansion trial examining avelumab for the treatment of patients with previously treated, recurrent or refractory ovarian cancer.

A team at Weill Cornell Medical College in New York that is studying ovarian cancer has not only discovered another mechanism by which tumors evade attack by the immune system, but is also devising a first-in-class potential treatment.

The glycoprotein CD31 was associated with improvements in overall survival and progression-free survival for patients with advanced ovarian cancer treated with frontline bevacizumab.

Robert L. Coleman, MD, FACOG, FACS, professor, Ann Rife Cox Chair in Gynecology, Department of Gynecologic Oncology and Reproductive Medicine, vice chair, Clinical Research, The University of Texas MD Anderson Cancer Center, discusses the results of the ARIEL2 trial, a phase II study to identify patients with ovarian cancer likely to respond to rucaparib using tumor genetic analysis.

Rucaparib showed an overall response rate of 82% among a cohort of patients with BRCA-mutated ovarian cancer in the phase II ARIEL2 study.

Patients with pretreated advanced ovarian cancer demonstrated encouraging signs of antitumor activity with monoclonal antibodies against programmed death-1 and its ligand PD-L1, according to findings from two clinical studies presented at the 2015 ASCO Annual Meeting.

The added overall survival benefit seen with intraperitoneal versus intravenous chemotherapy extends beyond 10 years for patients with ovarian cancer.

The oral combination of olaparib, a PARP inhibitor, and BKM120, a PI3K inhibitor, demonstrated to be safe and clinical beneficial in women with high-grade serous ovarian cancer, as well as patients with triple-negative breast cancer.

The treatment of patients with ovarian cancer has evolved from an approach focused on chemotherapy and surgery to one involving targeted therapies and monoclonal antibodies.

The FDA’s recent approval of olaparib for women with BRCA-mutated advanced ovarian cancer marks a significant therapeutic advance for women with the malignancy, but the specific indication is far too restrictive and the drug should be offered to many more patients.

Charles A. (Trey) Leath III, MD, associate professor, gynecologic oncology, University of Alabama Birmingham Cancer Center, discusses a study that examined clinical outcomes and quality of life in ovarian cancer.

Robert L. Coleman, MD, FACOG, FACS, professor, Department of Gynecologic Oncology and Reproductive Medicine, University of Texas MD Anderson Cancer Center, discusses a study which examined the impact of bevacizumab in ovarian cancer.
Despite their promise, checkpoint inhibitors are not effective in every patient, and research suggests the STING (stimulator of interferon genes) pathway may hold important clues as to why some tumors fail to respond.

To gain insight into this novel approach and its potential benefit, Targeted Oncology interviewed Bradley J. Monk, MD, FACOG, FACS, who presented phase II results at the 2015 SGO's Annual Meeting on Women's Cancer.

Sharyn N. Lewin, MD, discusses the importance of screening patients with ovarian cancer for a BRCA mutation.

The FDA has granted a breakthrough therapy designation to the PARP1/2 inhibitor rucaparib for the treatment of women with BRCA-mutated advanced ovarian cancer.

Intraperitoneal (IP) chemotherapy offers a median 10-month survival advantage over IV chemotherapy for women with advanced ovarian cancer. Result from a retrospective analysis were recently published in the Journal of Clinical Oncology.

In mid-December of 2014, the FDA approved the BRACAnalysis CDx diagnostic test to accompany the use of Lynparza (olaparib) in advanced ovarian cancer.
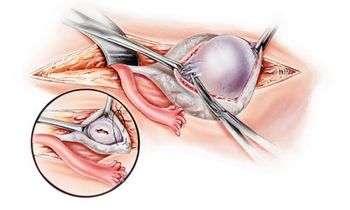
In a single-institution randomized prospective controlled trial of women with advanced epithelial ovarian cancer, 52.6% of patients in the PDS study arm had major complications with an MSKCC score of 3 or greater.

The addition fosbretabulin tromethamine to bevacizumab lowered the risk of progression by 31.5% but doubled the rate of hypertension compared with bevacizumab alone in pretreated patients with recurrent ovarian cancer enrolled in the phase II GOG186i study.

A triage algorithm may identify patients with presumed advanced ovarian cancer who represent preoperatively defined candidates for diagnostic laparoscopy.

Barbara Ann Goff, MD, discusses an analysis looking at the care of ovarian cancer.

Two newly reported studies are providing more insight into the efficacy and safety profile of the PARP inhibitor olaparib in ovarian cancer.

Bradley J. Monk, MD, FACOG, FACS, on bevacizumab in combination with the vascular disrupting agent fosbretabulin for the treatment of patients with recurrent ovarian, tubal, or peritoneal carcinoma.

The addition of bevacizumab to a doublet chemotherapy regimen extended OS by nearly 5 months compared with standard chemotherapy alone in women with platinum-sensitive recurrent ovarian cancers.






























