A phase III study of the investigational peptibody trebananib has met its primary endpoint of an improvement in progression-free survival.

Your AI-Trained Oncology Knowledge Connection!


A phase III study of the investigational peptibody trebananib has met its primary endpoint of an improvement in progression-free survival.

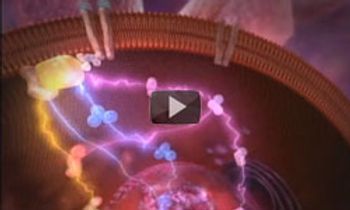
The mechanisms of the cMET signaling pathway and potential therapeutic implications. After binding with its ligand, cMET activates a wide range of different cellular signaling pathways.

Data from the French National Cancer Institute showed an increase in testing for BRCA1/2 for breast and ovarian cancer, though not for the MMR mutation for Lynch syndrome.
The FDA rejected a new drug application for tivozanib, an inhibitor of vascular endothelial growth factor (VEGF), for the treatment of advanced renal cell carcinoma (RCC).

The week of June 3 in review, featuring content on crizotinib in NSCLC, an FDA approval, novel agents for the treatment of prostate cancer, and more.

Lenalidomide has been approved to treat patients with mantle cell lymphoma who have relapsed or whose disease has progressed after two prior therapies including at least one prior treatment with bortezomib.

Crizotinib demonstrated superiority to chemotherapy for the treatment of previously treated, advanced non-small cell lung cancer (NSCLC) with ALK rearrangement in a trial published online by The New England Journal of Medicine.

The week of May 27 in review, featuring stories and videos on an FDA approval, T-DM1 for breast cancer, novel agents in early development, and the Notch pathway in gliomas.
The FDA approved both dabrafenib (Tafinlar) and trametinib (Mekinist) for the treatment of patients with metastatic or unresectable melanoma.

The week of May 20 in review, featuring stories and videos on biomarkers in prostate cancer, a phase III study halted after interim analysis, and priority review for Abraxane.
The FDA has granted priority review to paclitaxel protein-bound particles for injectable suspension, albumin-bound, or nab-paclitaxel, for metastatic pancreatic cancer when administered in combination with gemcitabine.

A phase III study of inotuzumab ozogamicin for patients with relapsed or refractory CD22+ aggressive non-Hodgkin lymphoma who are not candidates for high-dose chemotherapy was halted after a scheduled interim analysis.
Sorafenib is small molecule inhibitor approved for the treatment of primary kidney cancer and advanced primary liver cancer.

The phase III JAKARTA trial of SAR302503 for myelofibrosis met its primary endpoint in both dose groups, as reported by Sanofi.

Researchers have determined that high expression of microRNA-155 was associated with a poorer prognosis in patients with AML and that inhibition of a molecule that regulates the microRNA may serve as a therapeutic target for these patients.

The week of May 13 in review, featuring stories and videos on cabozantinib in medullary thyroid cancer, biomarkers in prostate cancer, and SAR302503 in myelofibrosis.
Abstracts published in the Annual Meeting Proceedings Part I featuring late-stage data on ibrutinib, eribulin, regorafenib, sipuleucel-T, lambrolizumab, palbociclib, and more.

ASCO highlighted studies on treatment with ipilimumab and nivolumab in advanced melanoma, radiation in NSCLC, and idelalisib in chronic lymphocytic leukemia.
Radium RA 223 dichloride has been approved by the FDA for the treatment of symptomatic metastatic castration-resistant prostate cancer (mCRPC) that has spread to the bones but not to any other organs.

The week of May 6 in review, featuring stories and videos on cabozantinib in medullary thyroid cancer, biomarkers in prostate cancer, and SAR302503 in myelofibrosis.

Acute myeloid leukemia (AML), the most common acute form of leukemia in adults, is potentially driven by at least one genetic mutation in nearly all cases.

Cetuximab is an epidermal growth factor receptor inhibitor used for the treatment of metastatic colorectal cancer and head and neck cancer.
Nearly three-fourths of patients with previously untreated advanced NSCLC with specific mutations of EGFR experienced one full year of PFS when given the investigational therapeutic agent dacomitinib.