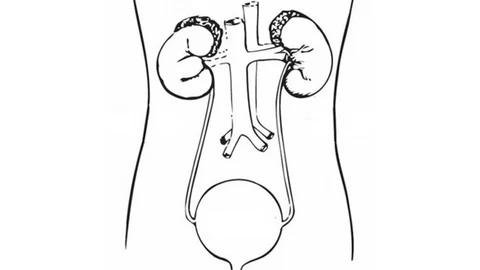
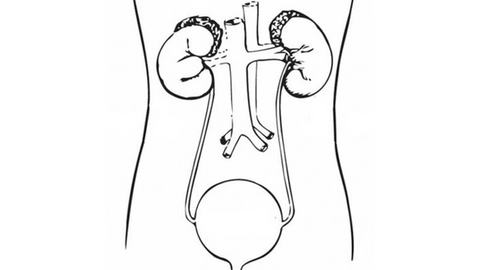
Bladder Cancer
Latest News
Latest Videos

More News

The FDA has granted fast track designation to belzupacap sarotalocan for the treatment of patients with non-muscle invasive bladder cancer. A phase 1 study is planned to launch in 2022.
In an interview with Targeted Oncology, Roger Li, MD, discussed the use of the CG0070 in combination with pembrolizumab and nivolumab for patients with non-muscle invasive bladder cancer who are unresponsive to Bacillus Calmette-Guerin.

In an interview with Targeted Oncology, Sumanta Pal, MD, PhD, discussed the COSMIC-021 study of cabozantinib and atezolizumab across multiple tumor types, including advanced urothelial carcinoma.

After 2-years in the phase 2 POLARIS-3 clinical trial, toripalimab demonstrated clinical activity in patients with urothelial carcinoma.

According to new COSMIC-021 data, atezolizumab combined with cabozantinib may be active in patients with urothelial carcinoma.


During an interview with Targeted Oncology, Shifeng S. Mao, MD, discussed what current and upcoming clinical studies have led to improvements in how physicians treat urothelial cancer.

A biologics license application has been submitted to the FDA for N-803 plus Bacillus Calmette-Guérin as treatment for patients with BCG-unresponsive non-muscle invasive bladder cancer in situ with or without Ta or T1 disease.
In an interview with Targeted Oncology, Joaquim Bellmunt, MD, PhD, discussed the long-term overall survival data for first-line maintenance avelumab in mUC presented at the AUA 2022 Annual Meeting and a sub-analysis of the JAVELIN Bladder 100 trial being read out at the upcoming 2022 ASCO Annual Meeting.
Thomas Flaig, MD, addressed new and emerging systemic therapies for bladder cancer during the National Comprehensive Cancer Network 2022 Annual Conference.

The combination of tislelizumab plus nab-paclitaxel demonstrated benefit and a tolerable safety profile in patients with high-risk non–muscle invasive bladder cancer.

Tislelizumab plus nab-paclitaxel as neoadjuvant treatment demonstrated benefit with a high rate of pathologic complete response in patients with muscle-invasive bladder cancer.

Both TROP-2 and Nectin-4 were shown to be highly expressed in urothelial cancer and variant histology bladder cancer, but not in patients with neuroendocrine histology.

Health-related quality-of-life outcomes were maintained among patients with low grade non-muscle invasive bladder cancer who were treated with UGN-102, a chemoablative reverse thermal gel as a primary approach in the single-arm phase 2b Optima II trial.

Interim results from a biomarker-informed preoperative study of infigratinib demonstrated substantial activity and tolerability in patients with localized upper tract urothelial carcinoma, according to findings of a phase 1b trial.

Chemotherapy plus intravenous vitamin C shows benefit in cisplatin-ineligible patients with locally advanced muscle-invasive bladder cancer.

Long-term follow-up results from the JAVELIN Bladder 100 study further support the standard-of-care role of avelumab as frontline maintenance in patients with urothelial carcinoma.

In patients with high-risk, muscle-invasive urothelial carcinoma treated in the adjuvant nivolumab lead to clinically meaningful improvements in disease-free survival compared with placebo.

Immune-related adverse effects may show significantly longer progression-free survival and overall survival in patients with metastatic urothelial carcinoma who are receiving pembrolizumab.

Matthew Galsky, MD, discussed the DS8201-A-U105 trial of T-DXd plus nivolumab in patients with HER2-expressing urothelial cancer.

Nintedanib plus neoadjuvant chemotherapy did not achieve improvement in pathologic complete response in patients with muscle invasive bladder cancer in the phase 2 NEOBLADE study, but the study provided important survival signals.
Adding pembrolizumab to CG0070 led to a complete response rate of 88.9% among 18 patients in the phase 2 CORE1 trial.

The phase 1 ADVANCED-1 trial of the biologic immunopotentiator agent TARA-002 began dosing patients with non-muscular invasive bladder cancer.
During a Targeted Oncology case-based roundtable event, Arlene Siefker-Radtke discussed the second-line treatment options for a patient with urothelial carcinoma and the EV-301 trial of enfortumab vedotin.

Following the important information provided by the phase 3 VESPER trial, Jean Hoffman-Censits, Md said there are several noteworthy ongoing phase 3 neoadjuvant immunotherapy studies in bladder cancer.