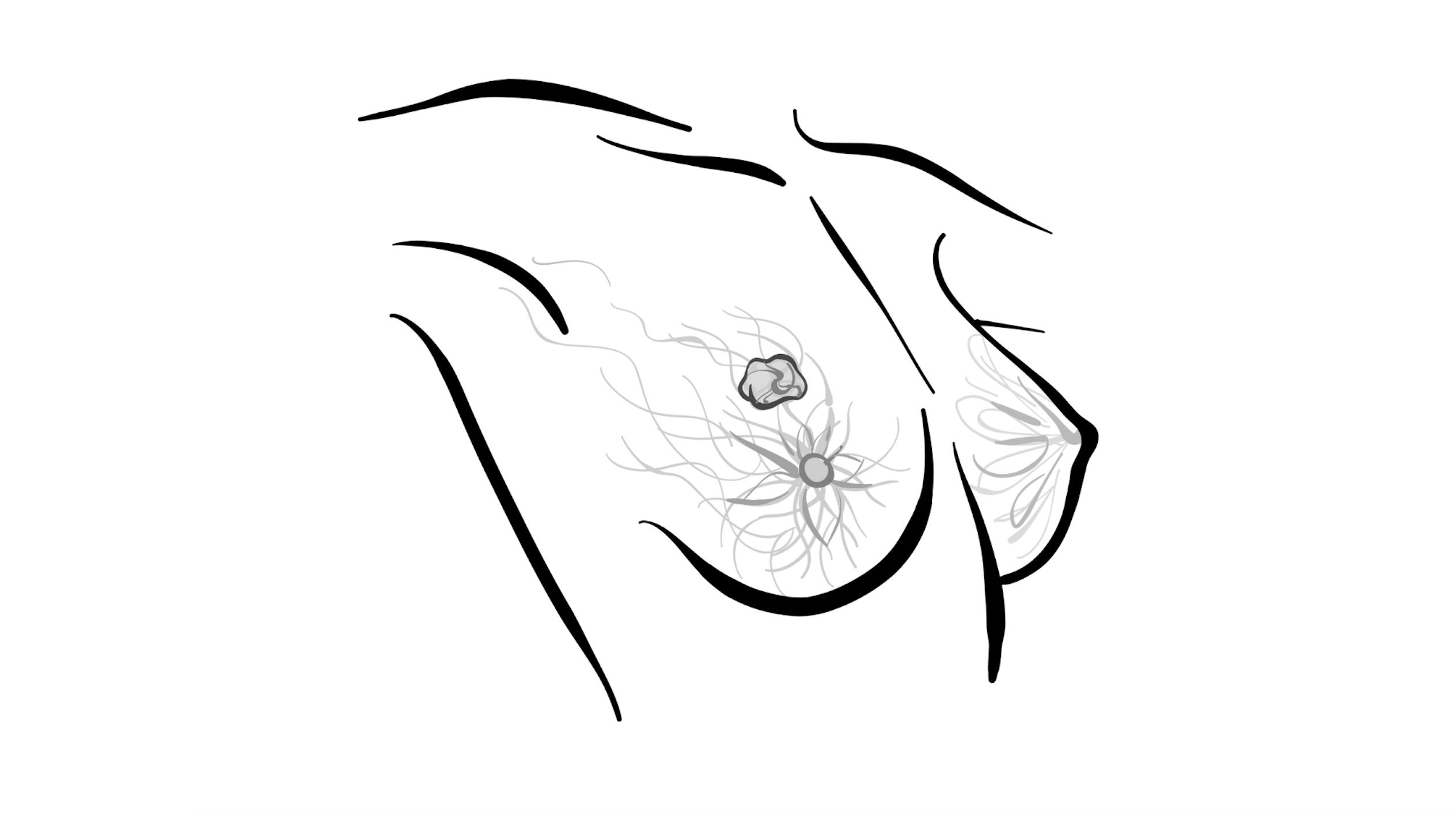
BREAST CANCER
Latest News
Latest Videos

More News

Pembrolizumab in the neoadjuvant and adjuvant settings demonstrated similar health-related quality-of-life scores in patients with triple-negative breast cancer when compared with placebo.

Patient-reported outcomes from the DESTINY-Breast04 trial showed a quality-of-life benefit from treatment with trastuzumab deruxtecan compared with physician’s choice of therapy in patients with hormone receptor–positive, HER2-low metastatic breast cancer.

The AMEERA-3 trial failed to meet its primary end point of superior progression-free survival for amcenestrant compared with endocrine therapy in patients with endocrine-resistant, ER-positive advanced breast cancer.

The antibody-drug conjugate sacituzumab govitecan demonstrated superior overall response rate and progression-free survival in patients with breast cancer with limited options due to lack of high HER2 expression.

While lasfoxifene alone did not meet the primary end points that researchers set out for it, they did find that it improves responses in patients with estrogen receptor–positive, HER2-negative metastatic breast cancer harboring ESR1 mutations.

The phase 2 DAWNA-2 trial of dalpiciclib plus letrozole or anastrozole led to a reduced the risk of disease progression vs chemotherapy alone in patients with treatment-naïve, hormone receptor–positive, HER2-negative advanced breast cancer.

Oleclumab plus durvalumab and chemotherapy did not increase clinical benefit rate for patients with advanced triple-negative breast cancer, according to results from the phase 2 SYNERGY trial.

Treatment with sactiuzumab govitecan improved overall survival, objective response rate, duration of response, and overall quality of life vs physician’s choice in the phase 3 TROPiCS-02 study.

Updated findings of the MONARCH 3 trial of abemaciclib added to a nonsteroidal aromatase inhibitor presented at ESMO 2022 revealed prolonged overall survival in hormone receptor-positive, HER2-negative breast cancer.

Sacituzumab govitecan demonstrated promising progression-free survival efficacy in patients with HR+/HER2-low and IHC0 status metastatic breast cancer, showing its potential as a future treatment option.

William J. Gradishar, MD, discusses the most recent evolutions in the management of patients with advanced breast cancer.

Hot flashes related to adjuvant hormone therapy were predictive of worse breast cancer outcomes, according to research on disease-free survival and treatment discontinuation in patients with and without hot flashes.

A case report for zenocutuzumab supports emerging evidence that NRG1 fusions are targetable driver mutations in metastatic breast cancer.
During a Targeted Oncology case-based roundtable event, Alison K. Conlin, MD, discussed with participants their choice of first- and second-line therapy for a patient with triple-negative breast cancer.

Patients with either breast cancer, endometrial cancer, or ovarian cancer have started treatment with XMT-1660 in a phase 1 clinical trial.

Sanofi has discontinued the clinical development program for amcenestrant due to unsatisfactory results from the phase 3AMEERA-5 trial.

During a live virtual event, Elizabeth Ann Mittendorf, MD, PhD, discussed the case of a patient with triple-negative breast cancer whose disease progressed following adjuvant dose-dense doxorubicin plus cyclophosphamide with paclitaxel, then frontline gemcitabine and carboplatin. This is the second of 2 articles based on this live event.

Positive findings from the phase 3 EMERALD study led to a new drug application for elacestrant as treatment of estrogen receptor-positive, HER2-negative advanced or metastatic breast cancer. The FDA has granted the application priority review.

Based on findings from the interim phase 1 portion of a trial examining ART4215 in combination with talazoparib in BRCA deficient breast cancer, a phase 2 portion will be initiated.

Evanthia Roussos Torres, MD, PhD, discusses the next steps and novel therapies currently showing promise in the HER-2 negative breast cancer space.

In an interview with Targeted OncologyTM, Debra Patt, MD, PhD, MBA, FASCO, discusses the impact of delayed cancer screenings in potential patients and how the COVID-19 pandemic has increased existing inequities in cancer care.

The FDA has approved fam-trastuzumab deruxtecan-nxki for the treatment of patients with unresectable or metastatic HER2-low breast cancer.

In an interview with Targeted Oncology, Jamie Brett and Seth Wander, MD, PhD, discussed recent findings regarding CDK4/6 for patients with hormone receptor-positive metastatic breast cancer with ESR1 alterations.

The phase 3 PADA-1 trial revealed a correlation between estrogen receptor- and progesterone receptor-positive metastatic breast cancer and prolonged progression-free survival.

Erika P. Hamilton, MD, discusses the evolution of fam-trastuzumab deruxtecan-nxki through its examination in various trials.