EGFR+ Lung Cancer
Latest News
Latest Videos

More News

Paul A. Bunn, Jr., MD, distinguished professor, Division of Medical Oncology, University of Colorado, James Dudley Chair in Lung Cancer Research, Lung Cancer: Giant of Cancer Care, discusses immunotherapy in lung cancer.

Walter J. Curran, Jr., MD, FACR, associate vice president of cancer, Woodruff Health Sciences Center, executive director, Winship Cancer Institute, Lawrence W. Davis Chair of Radiation Oncology, Emory University, discusses radiation therapy dose for the treatment of patients with lung cancer.

A new drug application (NDA) was recently submitted for rociletinib (CO-1686) as a treatment for patients who have EGFR T790M-positive metastatic non-small cell lung cancer (NSCLC) following previous administration of an EGFR TKI.

After a successful demonstration as single-agents, clinical trials are currently assessing PD-1 and PD-L1 inhibitors, combined with chemotherapy, targeted therapies, and radiation therapy, in an attempt to further improve outcomes for patients who have non-small cell lung cancer (NSCLC).

Barbara J. Gitlitz, MD, associate professor of clinical medicine, Keck Medicine, University of Southern California, discusses EGFR mutations in patients with lung cancer.

Next generation therapies proven to be highly effective, are in development for patients who have oncogene-driven non-small cell lung cancer (NSCLC), specifically those with alterations in EGFR, ALK, ROS1, and NTRK.

Roy Decker, MD, PhD, associate professor of therapeutic radiology, assistant professor of surgery (Otolaryngology), Clinical Research Program Leader, Therapeutic Radiology, Yale University, discusses stereotactic body radiation therapy (SBRT) in surgery for patients with early non-small cell lung cancer (NSCLC).
In addition to late patient presentation, the high mortality rate in lung cancer can be largely attributed to the complex nature of the disease and a very high rate of heterogeneity in the causative molecular abnormalities.

Immunotherapies (immune checkpoint inhibitors) and targeted therapies (tyrosine kinase inhibitors [TKIs] that target specific mutations in one or more oncogenic drivers) represent two of the most researched types of therapy under investigation for the treatment of non-small cell lung cancer (NSCLC).
An impressive array of newly approved treatments, as well as investigational agents, for non–small cell lung cancer (NSCLC) emerged in the first 6 months of 2015.

Martin Reck, MD, PhD, Head of Thoracic Oncology, Hospital Grosshansdorf, discusses the phase III SQUIRE trial in patients with non-small cell lung cancer (NSCLC) treated with necitumumab.

The University of Texas MD Anderson Cancer Center in Houston has been selected as the site for one of two new Genome Characterization Centers (GCCs) funded by the National Cancer Institute (NCI) and National Institute of Health (HHSN261200800001E).

Breakthrough therapy designation has been granted by the US Food and Drug Adminstration (FDA) to the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) as treatment for patients with BRAF V600E-mutant non–small cell lung cancer

Results from the phase III METEOR study showed that treatment with cabozantinib (Cometriq) improved progression-free survival (PFS) and overall survival (OS) compared with everolimus (Afinitor) in previously treated patients with metastatic renal cell carcinoma (mRCC).

Gefitinib (Iressa) was recently approved by the FDA for the frontline treatment of patients who have metastatic, EGFR-positive non-small cell lung cancer (NSCLC) with an exon 19 deletion or exon 21 (L858R) substitution.

Clovis Oncology has initiated the rolling submission process for rociletinib in EGFR T790M-positive advanced non–small cell lung cancer following prior treatment with a TKI.

Jennifer S. Temel, clinical director, Thoracic Oncology, Massachusetts General Hospital, discusses a phase III study that examined the impact of anamorelin in patients with advanced non-small cell lung cancer (NSCLC) and cachexia.
An assay that measures circulating tumor (ct) DNA in the urine can detect the early acquisition of epidermal growth factor receptor (EGFR) resistance mutations in patients with metastatic lung adenocarcinoma.
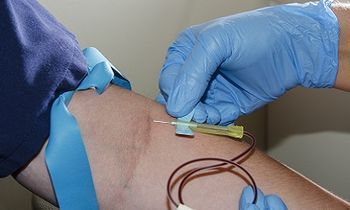
Researchers at the Wistar Institute Cancer Center have discovered a new potential circulating biomarker for non–small cell lung cancer. It is a cancer testis antigen expressed by a cancer/testis gene called AKAP4. The exciting prospect this heralds is the development of an accurate, quick blood test for early-stage NSCLC.

The next-generation ALK inhibitor alectinib has demonstrated robust objective response rates (ORR) in patients with ALK-positive non–small cell lung cancer, including those with central nervous system metastases.

An immunotherapy combination demonstrated an overall response rate of 27% in previously treated non–small cell lung cancer across a range of doses, according to results of an ongoing phase Ib study.

The FDA has granted a priority review to pembrolizumab as a potential treatment for patients with advanced non-small cell lung cancer following treatment with chemotherapy or a targeted therapy, if applicable.

Nivolumab (Opdivo) improved overall survival and was less toxic compared with docetaxel in chemotherapy-pretreated patients with nonsquamous non–small cell lung cancer.

MPDL3280A reduced the risk of death by 53% compared with docetaxel in previously treated patients with PD-L1-positive squamous and non-squamous non-small cell lung cancer.

D. Ross Camidge, MD, director, Thoracic Oncology Clinical Program, program director, Thoracic Oncology Clinical and Translational Research Fellowship, University of Colorado Denver, discusses MET as a secondary driver in lung cancer.