EGFR+ Lung Cancer
Latest News

Latest Videos

More News

A prospective study of retrospectively analyzed blood samples of patients with nonresectable pancreatic cancer found that plasma KRAS mutational burden correlates with overall survival (OS).

Pembrolizumab (Keytruda) achieved an overall response rate (ORR) of 45.2% among a cohort of patients with high PD-L1-expressing non–small cell lung cancer (NSCLC) in the phase I KEYNOTE-001 trial.

The availability of mutation-specific treatments and an increasing understanding of potential resistance mechanisms have provided immense opportunities for research and development of new therapies in lung cancer.
Abnormal driver mutations contribute to tumor progression and have been prime targets for many therapeutic studies in oncology; however, researchers are focusing more and more on the less-studied passenger mutations and their role in tumor progression.

Despite the availability of several new agents in the past 5 years for the treatment of melanoma, patients with advanced melanoma still suffer poor prognoses.

Thomas J. Lynch, MD, discusses the treatment of patients with T790M-mutant non-small cell lung cancer (NSCLC).
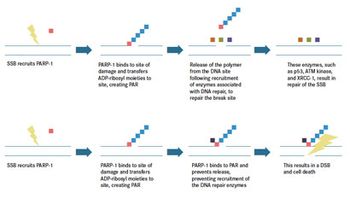
Lung cancer is one of the leading causes of death worldwide. The standard of care for advanced small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) includes platinumbased chemotherapy.
In a wide-ranging interview, Thomas Lynch, MD, provides expert insight across the spectrum of care, from screening to the challenges associated with resistance mutations.
Prior cancer history should not exclude patients with advanced lung cancer from participating in clinical trials because it does not impact outcomes, according to a study.

Heather Wakelee, MD, associate professor of medicine, oncology division, Stanford University, discusses the rationale for immunotherapy in non-small cell lung cancer (NSCLC).

Growth in healthcare spending in the United States continues to outpace growth in European countries that enjoy a similar standard of living.

Tumor genome profiling identifies driver mutations in breast tumors, however, it is still too early to use this information in clinical decision making, according to Hope S. Rugo, MD.

The FDA has approved nivolumab (Opdivo) for the treatment of patients with advanced non-small cell lung cancer (NSCLC). The approval comes 3 months ahead of the FDA’s scheduled decision date.
Nivolumab (Opdivo) has been granted a priority review for use in patients with previously treated, advanced, squamous non–small cell lung cancer (NSCLC).
Available therapies for patients with lung cancer are associated with a number of significant toxicities that must be effectively managed by oncologists.

Only about 5% of patients with cancer are recruited into oncology clinical trials, raising a concern that unless this improves, progress in the development new treatments for cancer may be delayed.

The definition of liquid biopsy has expanded to include the collection of cfDNA, along with various species of cell-free RNA and exosomes, all of which are capable of providing information on the disease status of cancer patients.

Early detection in lung cancer is strongly linked to better survival. In a landmark decision the CMS recently approved national coverage for lung cancer screening with LDCT for high-risk smokers.

Roman Perez-Soler, MD, chairman and chief, department of medical oncology, Montefiore Medical Center, deputy director, Albert Einstein Cancer Center, discusses the challenges oncologists still face in lung cancer.

Maria E. Arcila, MD, acting director, Diagnostic Molecular Pathology Laboratory, Memorial Sloan Kettering Cancer Center, discusses diagnostic platforms for lung cancer.

Blocking activin-A, a protein that is secreted by non-small cell lung cancer (NSCLC) cells, may prevent cancer metastasis, according to research conducted at the University of Virginia (UVA).

Many patients with non–small cell lung cancer (NSCLC) have no identifiable mutations, and therefore their disease cannot be managed with targeted treatments.
The oral multikinase inhibitor motesanib failed to meet the primary endpoint of improving progression-free survival (PFS) in patients with non–small cell lung cancer (NSCLC) in the phase III MONET-A trial.

Roy S. Herbst, MD, PhD, professor, Yale Cancer Center, chief of medical oncology, Smilow Cancer Hospital at Yale-New Haven, comments on the development of biomarkers for lung cancer.

An international team of scientists has shed additional light on the important role of Yes-associated protein (YAP) in tumor development and in treatment response.