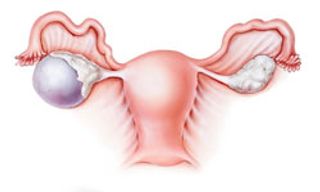
GYNECOLOGIC CANCERS
Latest News
Latest Videos

More News

Over the past two decades, there has been a shift away from indiscriminate cell-killing by anticancer agents toward the development of more specific drugs that target key aspects of cancer cell biology.

A phase III clinical program to investigate olaparib as a treatment for patients with BRCA-mutated ovarian cancer marks a step forward in the revived development of an agent that was once left on the sidelines.

Sandra Swain, MD, medical director of the Washington Cancer Institute at MedStar Washington Hospital Center, discusses the utility for vinegar as a screening tool in the U.S.
Measuring CA-125, the protein that predicts ovarian cancer recurrence, has shown promise as a screening tool for the disease.

Paul A. Bunn, Jr, MD, from the University of Colorado, discusses afatinib for patients with activating epidermal growth factor receptor mutation.

Carol Aghajanian, MD, from the Memorial Sloan-Kettering Cancer Center, discusses the difficulties with a gold standard clinical trial endpoint in ovarian cancer.

Pazopanib was better tolerated with noninferior efficacy when compared to sunitinib as a treatment for patients with advanced renal cell carcinoma.

A study shows that a new chemoresponse assay improves the overall survival and progression-free survival of patients with recurrent ovarian cancer while helping clinicians individualize care for those patients.

At the 18th Annual Conference of the National Comprehensive Cancer Network (NCCN), experts presented the latest updates to the NCCN Clinical Practice Guidelines in Oncology.

A quick reference chart of the key clinical trials presented at the American Society of Clinical Oncology (ASCO) 2013 Annual Meeting.

Electra D. Paskett, PhD, Professor, College of Medicine, The Ohio State University, discusses screening for cervical cancer.

Jane Robertson, MD, Global Product Vice President at AstraZeneca, describes the mechanism of action of the PARP inhibitor olaparib.

Antoni Ribas, MD, PhD, the director of the Tumor Immunology Program Area at UCLA's Jonsson Comprehensive Cancer Center, discusses PD-1 and PD-L1 in various cancers.

Richard Finn, MD, from the Jonsson Comprehensive Cancer Center, UCLA, describes the development of cyclin-dependent kinases (CDKs) for the treatment of cancer.

Researchers at the NCI have developed the most comprehensive analysis of coding variants in the most frequently studied human tumor cell lines in cancer research.
The risk of developing ovarian cancer has been linked to several factors including family history, age, and pregnancy history. In the U.S., ovarian cancer accounts for 3% of all cancer in women.

Krishnansu S. Tewari, MD, from the University of California, Irvine, comments on the next steps for bevacizumab for recurrent cervical cancer.
The next-generation PI3 kinase (PI3K) inhibitor GDC-0032 has shown signs of efficacy in patients with advanced cancers that were mutated for the PI3K alpha gene.

Andreas du Bois, MD, discusses the background, rationale, and results for the exploration of maintenance pazopanib as a treatment for patients with advanced ovarian cancer.

In advance of the 2013 ASCO Annual Meeting, Targeted Healthcare spoke with Mike Thompson, MD, PhD, about the use of social media in the field of oncology.

A phase III study of the investigational peptibody trebananib has met its primary endpoint of an improvement in progression-free survival.

Data from the French National Cancer Institute showed an increase in testing for BRCA1/2 for breast and ovarian cancer, though not for the MMR mutation for Lynch syndrome.

Krishnansu S. Tewari, MD, describes a phase III trial conducted by the Gynecologic Oncology Group exploring treatment with bevacizumab for patients with recurrent cervical cancer.

Electra D. Paskett, PhD, Professor, College of Medicine, The Ohio State University, describes the use of vinegar (acetic acid) as a cervical cancer screening tool.

Carol Aghajanian, MD, from the Memorial Sloan-Kettering Cancer Center, discusses a phase III trial of pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.