
LUNG CANCER
Latest News

Breakthrough therapy designation has been granted by the US Food and Drug Adminstration (FDA) to the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) as treatment for patients with BRAF V600E-mutant non–small cell lung cancer
Latest Videos

More News

The approval of necitumumab, combined with gemcitabine and cisplatin for the first-line treatment of patients who have locally advanced or metastatic squamous non-small cell lung cancer (NSCLC), was supported by FDA’s Oncologic Drugs Advisory Committee (ODAC).

James Stevenson, MD, Department of Hematology and Oncology, Cleveland Clinic, discusses the impact of a stage IV non-small cell lung cancer (NSCLC) care pathway on frontline and maintenance chemotherapy use.

Clovis Oncology has initiated the rolling submission process for rociletinib in EGFR T790M-positive advanced non–small cell lung cancer following prior treatment with a TKI.

Jennifer S. Temel, clinical director, Thoracic Oncology, Massachusetts General Hospital, discusses a phase III study that examined the impact of anamorelin in patients with advanced non-small cell lung cancer (NSCLC) and cachexia.

Roy S. Herbst, MD, PhD, Ensign Professor of Medicine (medical oncology), professor of Pharmacology, chief, Medical Oncology, Yale Cancer Center and Smilow Cancer Hospital at Yale-New Haven, associate director, Translational Research, Translational Working Group Leader, Thoracic Oncology Program, Yale Cancer Center, discusses the results of the CheckMate-057 trial, which examined nivolumab in patients with NSCLC, and possible biomarkers that could be identified.
Researchers at the Wistar Institute Cancer Center have discovered a new potential circulating biomarker for non–small cell lung cancer. It is a cancer testis antigen expressed by a cancer/testis gene called AKAP4. The exciting prospect this heralds is the development of an accurate, quick blood test for early-stage NSCLC.

Veena Singh, MD, senior vice president and senior medical director, Biocept, Inc., discusses the clinical validation and utility of a liquid biopsy in non-small cell lung cancer (NSCLC) using circulating tumor cells (CTCs) and ctDNA to analyze EGFR, ALK and ROS status.

The next-generation ALK inhibitor alectinib has demonstrated robust objective response rates (ORR) in patients with ALK-positive non–small cell lung cancer, including those with central nervous system metastases.

An immunotherapy combination demonstrated an overall response rate of 27% in previously treated non–small cell lung cancer across a range of doses, according to results of an ongoing phase Ib study.

The FDA has granted a priority review to pembrolizumab as a potential treatment for patients with advanced non-small cell lung cancer following treatment with chemotherapy or a targeted therapy, if applicable.

Single-agent atezolizumab (formerly MPDL3280A) had excellent clinical activity in both chemotherapy-naïve and previously treated patients with metastatic non–small cell lung cancer (NSCLC) in the phase II FIR study,

Nivolumab (Opdivo) improved overall survival and was less toxic compared with docetaxel in chemotherapy-pretreated patients with nonsquamous non–small cell lung cancer.

MPDL3280A reduced the risk of death by 53% compared with docetaxel in previously treated patients with PD-L1-positive squamous and non-squamous non-small cell lung cancer.

D. Ross Camidge, MD, director, Thoracic Oncology Clinical Program, program director, Thoracic Oncology Clinical and Translational Research Fellowship, University of Colorado Denver, discusses MET as a secondary driver in lung cancer.

Edward S. Kim, MD, chairman, Solid Tumor Oncology and Investigational Therapeutics, Levine Cancer Institute, Carolinas HealthCare System, discusses combining bevacizumab with EGFR tyrosine kinase inhibitors (TKI) in the treatment of lung cancer.

John Heymach, MD, PhD, chair of Thoracic/Head and Neck Medical Oncology, MD Anderson Cancer Center, discusses the characteristics of three more KRAS subsets in lung cancer, which researchers discovered in a recent study.

Leaders of Roswell Park Cancer Institute (RPCI) in Buffalo, New York, have agreed to collaborate with the Center for Molecular Immunology (CIM) in Cuba to evaluate a therapeutic anticancer vaccine for non–small cell lung cancer (NSCLC) in the United States.

The third-generation EGFR inhibitor rociletinib demonstrated promising responses in patients with EGFR-mutated non-small cell lung cancer with or without the acquired resistance mutation T790M.
Treatment with the third-generation EGFR inhibitor AZD9291 was found to be highly active in patients with EGFR T790M-mutated NSCLC following progression on prior therapy with an EGFR TKI.
Despite their promise, checkpoint inhibitors are not effective in every patient, and research suggests the STING (stimulator of interferon genes) pathway may hold important clues as to why some tumors fail to respond.

Pembrolizumab (Keytruda) achieved an overall response rate (ORR) of 45.2% among a cohort of patients with high PD-L1-expressing non–small cell lung cancer (NSCLC) in the phase I KEYNOTE-001 trial.

The availability of mutation-specific treatments and an increasing understanding of potential resistance mechanisms have provided immense opportunities for research and development of new therapies in lung cancer.

Thomas J. Lynch, MD, discusses the treatment of patients with T790M-mutant non-small cell lung cancer (NSCLC).
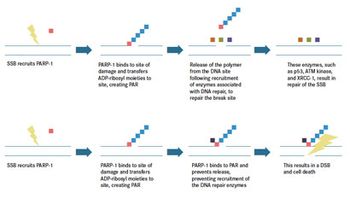
Lung cancer is one of the leading causes of death worldwide. The standard of care for advanced small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) includes platinumbased chemotherapy.
In a wide-ranging interview, Thomas Lynch, MD, provides expert insight across the spectrum of care, from screening to the challenges associated with resistance mutations.


















































