
While the concept of cancer-specific immunotherapy is not new, it recently has been proven feasible as a rational treatment for patients with some of the most challenging and difficult malignancies.

Your AI-Trained Oncology Knowledge Connection!


While the concept of cancer-specific immunotherapy is not new, it recently has been proven feasible as a rational treatment for patients with some of the most challenging and difficult malignancies.

Harriet Kluger, MD, associate professor of medicine (medical oncology), associate director, Hematology/Oncology Fellowship Program, Yale Cancer Center, explains how immunotherapies are changing the treatment of melanoma.

Until recently, the cornerstone of therapy for metastatic melanoma had been chemotherapy with dacarbazine (DTIC) and immunotherapy with high-dose interleukin-2 (HD IL-2) or interferon- (IFN- ).

Antoni Ribas, MD, PhD, professor of medicine, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, discusses the excitement surrounding immunotherapies for the treatment of patients with melanoma.

Georgina Long, BSc, PhD, MBBS, FRACP, medical oncologist, translational researcher, Melanoma Institute Australia, The University of Sydney, highlights targeted therapies in development for melanoma.

Skin cancer is the most common form of malignancy in the United States, and melanoma represents the most deadly subset.
Updates on dabrafenib, trametinib, lambrolizumab, and more.
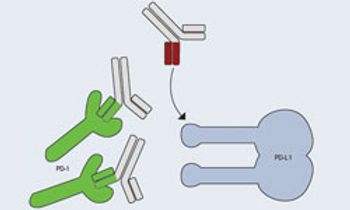
There are currently numerous experimental therapeutic options in various phases of clinical development that may hold promise for patients with advanced melanoma.

Merck announced the signing of three separate clinical collaboration agreements to evaluate the potential of MK-3475 across multiple tumor types. The agreements were signed through subsidiaries with Amgen Inc., Incyte Corporation, and Pfizer Inc.

Harriet Kluger, MD, associate professor of medicine (medical oncology), associate director, Hematology/Oncology Fellowship Program, Yale Cancer Center, comments on the changing landscape of immunotherapies for the treatment of melanoma.

The combination of the MEK inhibitor trametinib (Mekinist) and the BRAF inhibitor dabrafenib (Tafinlar) has received an accelerated approval from the FDA as a treatment for patients with unresectable or metastatic melanoma who harbor a BRAF V600E or V600K mutation.

The discovery of two unique molecular targets known to speed skin-cancer growth has researchers excited that they might soon understand and develop genetic therapies geared toward some of medicine’s most untreatable melanomas.

Most cases of BCC are curable with such approaches as surgery and radiation. In some cases, however, BCC can progress to a point of local invasion for which surgery and radiation therapy are not indicated.

Although the diagnosis of late-stage melanoma is still associated with a poor prognosis, an encouraging number of new therapies have been developed during the last 3 years.

TargetedOnc.com interviewed senior member Jeffrey S Weber, MD, PhD, from the Moffitt Cancer Center, University of South Florida, Tampa.

Although basal cell carcinoma (BCC) is the most common type of skin cancer, it typically does not advance beyond locally contained disease.

Antoni Ribas, MD, PhD, professor of medicine, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, discusses the outlook for BRAF inhibitors in melanoma.

At the 10th International Meeting of the Society for Melanoma Research, researchers provided insight into combination treatments and immunotherapies for the treatment of melanoma.

Georgina Long, BSc, PhD, MBBS, FRACP, medical oncologist, translational researcher, Melanoma Institute Australia, The University of Sydney, discusses the treatment of melanoma with emerging targeted therapies.

Adil Daud, MD, clinical professor, medicine, dermatology, University of California, San Francisco, discusses the challenges of treating patients with metastatic melanoma with BRAF inhibitor monotherapy.

Harriet Kluger, MD, associate professor of medicine (medical oncology), associate director, Hematology/Oncology Fellowship Program, Yale Cancer Center, discusses the difference between blocking PD-1 and PD-L1 in melanoma.

T-VEC, a novel oncolytic immunotherapy derived from the herpes simplex virus type 1, demonstrated a significant improvement in DRR, the primary endpoint in a pivotal phase III trial in patients with stage IIIB-IV melanoma.

Concurrent nivolumab and ipilimumab produced “rapid and deep†responses in patients with advanced melanoma who took part in the first phase I trial to evaluate the PD-1-blocking antibody nivolumab, along with the CTLA-4-blocking antibody ipilimumab.
The investigational anti-PD-1 immunotherapy MK-3475 has demonstrated an overall survival rate of 81% at one year in patients with advanced melanoma.

Ragini Kudchadkar, MD, discusses the benefits of combining BRAF and MEK inhibitors when treating melanoma.