
Knowledge of genetic expression of melanocytic lesions significantly reduced the number of indeterminate diagnoses made by dermatopathologists.

Your AI-Trained Oncology Knowledge Connection!


Knowledge of genetic expression of melanocytic lesions significantly reduced the number of indeterminate diagnoses made by dermatopathologists.

Pembrolizumab (Keytruda) significantly improved progression-free survival (PFS) by over 43% compared with chemotherapy as a treatment for patients with metastatic melanoma who were refractory to ipilimumab (Yervoy).

Frontline treatment with nivolumab (Opdivo) significantly extended overall survival (OS) and progression-free survival (PFS) when compared with dacarbazine in patients with untreated BRAF wild-type advanced melanoma.

Several novel approaches to the delivery of therapeutics for advanced melanoma may improve treatment efficacy and patient survival.

Adding the GM-CSF agent sargramostim to the CTLA-4 inhibitor ipilimumab (Yervoy) prolonged overall survival (OS) and lowered toxicity for patients with unresectable stage III or IV melanoma.
The FDA has expanded the approval of Lymphoseek (technetium Tc 99m tilmanocept) injection to include sentinel lymph node (SLN) detection for breast cancer and melanoma as well as lymphatic mapping in solid tumors.
Nanotechnology is unlikely to enter the arena of melanoma therapy, overshadowed as it is by the promising developments in combination treatment with immune checkpoint inhibitors, tumor-infiltrating lymphocyte therapies, and other immunotherapies.

Harriet Kluger, MD, associate professor of medicine, Yale Cancer Center, discusses the toxicity profile of concurrent nivolumab and ipilimumab as seen in a phase I trial.

Patients with unresectable melanoma had a significant improvement in the odds of survival when treated in first line with a combination of BRAF and MEK inhibitors, as opposed to anti-BRAF monotherapy.

Treatment with nivolumab (Opdivo) demonstrated superior objective response rates (ORR) and longer durations of response compared with chemotherapy in a phase III trial of patients with previously treated advanced metastatic melanoma

The FDA has assigned a priority review designation to nivolumab for pretreated patients with advanced melanoma, setting an action date for the drug as March 30, 2015.

Lawrence Fong, MD, discusses the systemic antitumor effect and clinical response in a phase II trial of intratumoral electroporation of plasmid interleukin-12 (IL-12) in patients with advanced melanoma.

Of the many signaling cascades being targeted for therapeutic intervention in cancer, one of the most important and best understood is the MAPK pathway, particularly the RAS/RAF/MEK/ERK cascade.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the potential to partner T-VEC with a targeted agent for the treatment of patients with melanoma.

Several advances in the treatment of metastatic melanoma have occurred in the last 5 years, one of which has been the approval by the FDA of targeted treatments for patients with melanomas harboring a BRAF mutation.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses the potential for immunotherapy combinations for the treatment of melanoma.

Unlike many other types of tumors, melanoma can be detected early in its progression by visual, noninvasive methods.
Only in the last 2 decades or so have sufficient data become available to statistically associate high mitotic rate with survival in melanomas.

Last week, the FDA approved pembrolizumab (Keytruda) for the treatment of patients with advanced or unresectable melanoma following progression on prior therapies.

Since the FDA approval of ipilimumab, there has been considerable interest in the potential for other immune system checkpoints, such as PD-L1 and its receptor, PD-1, to serve as therapeutic targets in metastatic melanoma.

Bristol-Myers Squibb has filed a lawsuit over Merck’s newly approved immunotherapy drug pembrolizumab, contending that the much-heralded PD-1 inhibitor will infringe upon patents that Bristol-Myers holds on the groundbreaking technology.

The FDA has approved pembrolizumab (Keytruda) as a treatment for patients with advanced or unresectable melanoma following progression on prior therapies.

Recent advances in immunotherapy and in agents that target specific genetic mutations in the mitogen-activated protein kinase (MAPK) pathway have led to dramatic improvements in outcomes for patients with advanced cutaneous melanoma.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the rationale behind a phase Ib study to evaluate the efficacy and safety of T-VEC and ipilimumab in previously untreated, unresected stage IIIB-IV melanoma
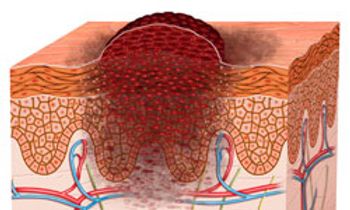
Risk factors for contracting melanoma are complex and can include environmental exposure and an individual’s genetic susceptibility, but people at highest risk are those with a personal or family history of melanoma and those with large, atypical, and/or multiple (more than 50) nevi.