
The FDA has assigned a priority review designation to bevacizumab (Avastin) in combination with chemotherapy for patients with recurrent platinum-resistant ovarian cancer.

Your AI-Trained Oncology Knowledge Connection!


The FDA has assigned a priority review designation to bevacizumab (Avastin) in combination with chemotherapy for patients with recurrent platinum-resistant ovarian cancer.

The somatostatin analog lanreotide (Somatuline Depot) significantly prolonged progression-free survival (PFS) compared with placebo for patients with metastatic gastroenteropancreatic NETs.
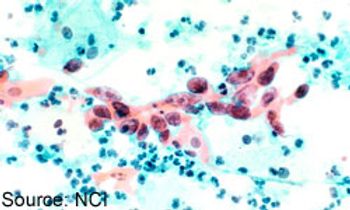
The FDA has assigned a priority review designation to bevacizumab (Avastin) plus chemotherapy as a treatment for patients with persistent, recurrent, or metastatic cervical cancer.

A phase III study comparing trametinib (Mekinist) plus dabrafenib (Tafinlar) with single-agent vemurafenib (Zelboraf) has been stopped early following a positive interim analysis.
The combination of the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib significantly improved progression-free survival (PFS) compared with vemurafenib alone for patients with untreated BRAFV600-mutated advanced melanoma.

The investigational CD19-targeted chimeric antigen receptor (CAR) therapy CTL019 has received a breakthrough therapy designation from the FDA as a potential treatment for pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

The PD-1 inhibitor nivolumab and the second-generation ALK inhibitor alectinib have each gained their first approvals as treatments for patients in Japan.

The FDA has approved the novel pan-HDAC inhibitor belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma.

The FDA has granted a breakthrough therapy designation to blinatumomab for the treatment of adult patients with Philadelphia-negative relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL).
For the prevention of nausea and vomiting after the first day of cisplatin-based chemotherapy, both aprepitant and metoclopramide when used in combination with dexamethasone were found to be similarly effective.
Four independent prognostic factors for improved overall survival (OS) in patients with locally advanced pancreatic cancer (LAPC) emerged from an analysis of the LAP 07 phase III trial.

The FDA’s ODAC voted 11-2 against the accelerated approval of the PARP inhibitor olaparib as a maintenance therapy for women with platinum-sensitive relapsed ovarian cancer with germline BRCA mutations.
Frontline treatment with the anti-PD-1 agent nivolumab significantly extended overall survival (OS) when compared with dacarbazine for patients with metastatic or unresectable melanoma.

Triple-negative breast cancer (TNBC) accounts for only 15% to 20% of breast cancers in the US.

Continuing EGFR inhibition beyond progression with afatinib (Gilotrif) plus paclitaxel significantly improved PFS and ORR compared with chemotherapy alone in patients with heavily pretreated metastatic NSCLC.
This activity is designed to educate oncologists and oncology nurses on evolving strategies for management of early-stage and metastatic triple-negative breast cancer.
The FDA has approved gadobutrol (Gadavist) injection for intravenous use with breast MRI to detect and identify the extent of disease.

The FDA has approved the radioactive diagnostic imaging agent Lymphoseek injection to guide sentinel lymph node biopsy in patients with cancer of the head and neck.
The phase III REACH trial, which examined ramucirumab (Cyramza) for the second-line treatment of patients with hepatocellular carcinoma (HCC), failed to meet its primary endpoint of overall survival (OS).
Nivolumab and elotuzumab have each been granted breakthrough therapy designations by the FDA for the treatment of two types of blood cancers.

The ASCO clinical practice guideline now recommends treatment with adjuvant tamoxifen for 10 years in women with stage I-III hormone receptor (HR)-positive breast cancer.

WETA, the Washington, DC, public television station, has launched a new website as part of a national outreach for the documentary The Story of Cancer: The Emperor of All Maladies, which will be produced by Ken Burns.

The FDA has approved panitumumab (Vectibix) in combination with chemotherapy as a frontline treatment for patients with <em>KRAS</em> wild-type metastatic colorectal cancer (mCRC), based on findings from two phase III clinical trials.

A cell-cycle gene array test demonstrated independent value for predicting metastatic progression after surgery for organ-confined renal cell carcinoma (RCC) of clear cell histology.
The EGFR inhibitor CO-1686 has received a breakthrough therapy designation from the FDA for its potential as a treatment for patients with metastatic T790M mutation-positive non-small cell lung cancer (NSCLC) who have received at least one prior line of EGFR-targeted therapy.

A phase III study exploring the chemotherapy regimen DHAP plus ofatumumab failed to meet its primary endpoint of prolongation in PFS when compared with DHAP plus rituximab for patients with relapsed or refractory DLBCL.

Despite improved efficacy with new therapies, both as monotherapy or in combinations, they provide new challenges to nurses in managing side effects and adjusting treatment.
Treatment with the investigational oral agent TAS-102 significantly improved overall survival (OS) in a phase III trial for patients with heavily pretreated metastatic colorectal cancer (mCRC).

The combination of vemurafenib with the investigational MEK inhibitor cobimetinib demonstrated a 13.7-month median PFS and an ORR of 87% in treatment-naïve patients with BRAFV600 mutation-positive metastatic melanoma.

The FDA has assigned a priority review designation to the PD-1 inhibitor pembrolizumab (MK-3475) as a treatment for patients with unresectable or metastatic melanoma following progression on ipilimumab.