
Renal Cell Carcinoma
Latest News

Latest Videos

More News
The risk of death for patients with advanced renal cell carcinoma (RCC) was reduced by 27% with nivolumab (Opdivo) versus everolimus (Afinitor), and overall survival (OS) was improved by 5.4 months.

The PD-1 inhibitor immunotherapy Nivolumab (Opdivo) has received an FDA breakthrough therapy designation for the treatment of patients with advanced renal cell carcinoma.

Cabozantinib (Cometriq) was given a breakthrough therapy designation by the FDA for the treatment of patients who have advanced renal cell carcinoma (RCC), following one prior therapy.

Lenvatinib (Lenvima) was recently granted a breakthrough therapy designation by the FDA as a potential treatment for patients with advanced renal cell carcinoma (RCC) who have received a VEGF-targeted therapy.

An independent panel has halted the phase III CheckMate-025 trial after determining that nivolumab (Opdivo) improved overall survival versus everolimus (Afinitor) in patients with advanced renal cell carcinoma.

Sunitinib treatment is associated with a significantly increased risk of all-grade and high-grade hypertension.

Lenvatinib alone and in combination with everolimus significantly improved progression-free survival compared with everolimus alone in a phase II study of patients with metastatic renal cell carcinoma.

Treatment with the PD-1 inhibitor nivolumab was active and tolerable across three doses for patients with metastatic renal cell carcinoma.
Despite their promise, checkpoint inhibitors are not effective in every patient, and research suggests the STING (stimulator of interferon genes) pathway may hold important clues as to why some tumors fail to respond.

Mario Sznol, MD, professor of medicine, Yale Cancer Center, discusses the role of high-dose IL-2 for the treatment of renal cell carcinoma (RCC).

Growth in healthcare spending in the United States continues to outpace growth in European countries that enjoy a similar standard of living.

Treatment with 2 angiogenesis-targeting drugs led to objective responses in a fourth of patients with advanced renal cell carcinoma (RCC), results of a dose-finding study showed.

MPDL3280A, an investigational antibody that targets programmed death-ligand 1 (PD-L1), in combination with bevacizumab had strong antitumor activity and induced responses in 4 of 10 patients with mRCC.

Martin H. Voss, MD, medical oncologist, Memorial Sloan Kettering Cancer Center, discusses the phase I/II DART study.

Neither sorafenib nor sunitinib improved outcomes when administered after surgery to patients with locally advanced renal cell carcinoma (RCC), according to results from the phase III ASSURE trial. These results were presented at a presscast held ahead of the 2015 Genitourinary Cancers Symposium.

Representatives Diana DeGette (D, Colorado) and Fred Upton (R, Michigan) recently released a "discussion draft" of the 21st Century Cures Act.
Metastatic disease accounts for the vast majority of cancer-related deaths. Ensuring a definitive diagnosis and the most effective treatment in a timely fashion is essential for extending life expectancy.

Amgen and Kite Pharma have announced that they will collaborate on the development of novel CAR T-cell immunotherapies, with Amgen providing cancer targets and Kite offering its engineered autologous cell therapy platform.

The anti-CD19 chimeric antigen receptor (CAR)-modified T-cell therapy CTL019 demonstrated a 92% complete response (CR) rate in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

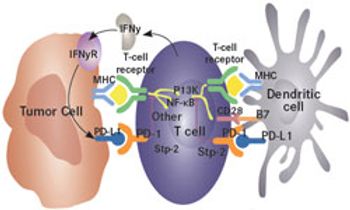
The programmed death receptor-1 (PD-1) ligand, PD-L1, has become a viable target for immunotherapy in cancer, with multiple antibodies now in development.
It is estimated that 1 in 63 individuals in the United States will develop renal cell carcinoma (RCC), making it among the most common cancers in the country.
As its CAR T cell and high-affinity TCR products continue to advance in clinical trials, Juno Therapeutics, Inc, filed a registration statement for an initial public offering (IPO) of its common stock on November 17.

Recent news stories profiling a cancer patient whose last hope rests on treatment by injections of the virus that causes AIDS may have created some misconceptions regarding a new cancer immunotherapy.

Robert J. Motzer, MD, attending physician, Memorial Sloan Kettering Cancer Center, discusses nivolumab for the treatment of metastatic renal cell carcinoma.

Thomas E. Hutson, DO, PharmD, medical oncologist, Texas Oncology–Baylor Charles A. Sammons Cancer Center, discusses the rationale behind the ATLAS Study, a randomized double-blind phase III study of adjuvant axitinib versus placebo in subjects at high risk of recurrent renal cell carcinoma (RCC).



















































