SKIN CANCERS
Latest News
Latest Videos

More News

Patients with unresectable melanoma had a significant improvement in the odds of survival when treated in first line with a combination of BRAF and MEK inhibitors, as opposed to anti-BRAF monotherapy.

Treatment with nivolumab (Opdivo) demonstrated superior objective response rates (ORR) and longer durations of response compared with chemotherapy in a phase III trial of patients with previously treated advanced metastatic melanoma

The FDA has assigned a priority review designation to nivolumab for pretreated patients with advanced melanoma, setting an action date for the drug as March 30, 2015.

Lawrence Fong, MD, discusses the systemic antitumor effect and clinical response in a phase II trial of intratumoral electroporation of plasmid interleukin-12 (IL-12) in patients with advanced melanoma.

Of the many signaling cascades being targeted for therapeutic intervention in cancer, one of the most important and best understood is the MAPK pathway, particularly the RAS/RAF/MEK/ERK cascade.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the potential to partner T-VEC with a targeted agent for the treatment of patients with melanoma.

Several advances in the treatment of metastatic melanoma have occurred in the last 5 years, one of which has been the approval by the FDA of targeted treatments for patients with melanomas harboring a BRAF mutation.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses the potential for immunotherapy combinations for the treatment of melanoma.

Unlike many other types of tumors, melanoma can be detected early in its progression by visual, noninvasive methods.
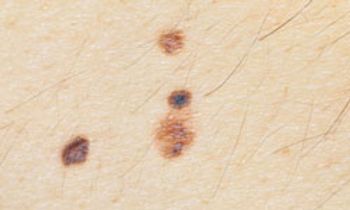
Only in the last 2 decades or so have sufficient data become available to statistically associate high mitotic rate with survival in melanomas.

Last week, the FDA approved pembrolizumab (Keytruda) for the treatment of patients with advanced or unresectable melanoma following progression on prior therapies.

Since the FDA approval of ipilimumab, there has been considerable interest in the potential for other immune system checkpoints, such as PD-L1 and its receptor, PD-1, to serve as therapeutic targets in metastatic melanoma.

Bristol-Myers Squibb has filed a lawsuit over Merck’s newly approved immunotherapy drug pembrolizumab, contending that the much-heralded PD-1 inhibitor will infringe upon patents that Bristol-Myers holds on the groundbreaking technology.

The FDA has approved pembrolizumab (Keytruda) as a treatment for patients with advanced or unresectable melanoma following progression on prior therapies.

Recent advances in immunotherapy and in agents that target specific genetic mutations in the mitogen-activated protein kinase (MAPK) pathway have led to dramatic improvements in outcomes for patients with advanced cutaneous melanoma.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the rationale behind a phase Ib study to evaluate the efficacy and safety of T-VEC and ipilimumab in previously untreated, unresected stage IIIB-IV melanoma
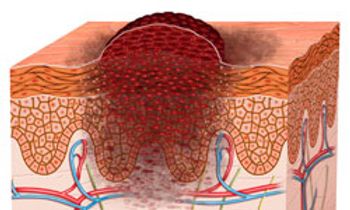
Risk factors for contracting melanoma are complex and can include environmental exposure and an individual’s genetic susceptibility, but people at highest risk are those with a personal or family history of melanoma and those with large, atypical, and/or multiple (more than 50) nevi.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses a phase I trial that examined the combination of nivolumab and ipilimumab for the treatment of advanced melanoma. The data presented at the 2014 ASCO Annual Meeting was an updated survival and clinical activity analysis in initially enrolled cohorts and activity by BRAF mutation status.

A phase III study comparing trametinib (Mekinist) plus dabrafenib (Tafinlar) with single-agent vemurafenib (Zelboraf) has been stopped early following a positive interim analysis.
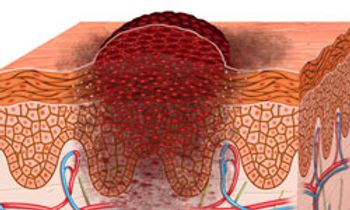
The combination of the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib significantly improved progression-free survival (PFS) compared with vemurafenib alone for patients with untreated BRAFV600-mutated advanced melanoma.

The investigational CD19-targeted chimeric antigen receptor (CAR) therapy CTL019 has received a breakthrough therapy designation from the FDA as a potential treatment for pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

The PD-1 inhibitor nivolumab and the second-generation ALK inhibitor alectinib have each gained their first approvals as treatments for patients in Japan.

Loren Clarke, MD, vice president of Medical Affairs of Dermatology at Myriad Genetics, discusses the company’s new myPath test for melanoma
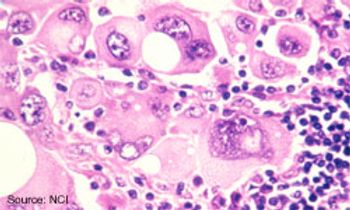
Frontline treatment with the anti-PD-1 agent nivolumab significantly extended overall survival (OS) when compared with dacarbazine for patients with metastatic or unresectable melanoma.

Jedd D. Wolchok, MD, PhD, discusses an updated analysis presented at the 2014 ASCO Annual Meeting that looked at pembrolizumab (MK-3475) for patients with melanoma.