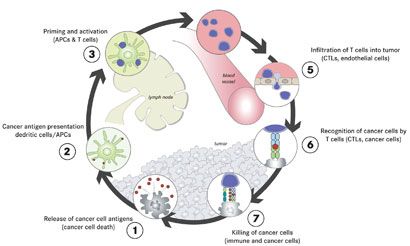
SKIN CANCERS
Latest News
Latest Videos

More News

The FDA has accepted a supplemental Biologics License Application (sBLA) for ipilimumab (Yervoy) as an adjuvant treatment of patients with stage III melanoma at high risk of recurrence following complete resection.

The MEK inhibitor cobimetinib has received an FDA priority review for use in combination with the BRAF inhibitor vemurafenib (Zelboraf) to treat patients with BRAF V600–positive advanced melanoma.

An international team of scientists has shed additional light on the important role of Yes-associated protein (YAP) in tumor development and in treatment response.

The FDA has scheduled a hearing to discuss the biologics license application (BLA) for the immunotherapy talimogene laherparepvec (T-VEC) as a treatment for patients with metastatic melanoma.

The risk of death was significantly reduced for patients with BRAFV600E/K mutation-positive metastatic melanoma treated with dabrafenib and trametinib compared with dabrafenib alone.

Representatives Diana DeGette (D, Colorado) and Fred Upton (R, Michigan) recently released a "discussion draft" of the 21st Century Cures Act.

Epigenetic and genetic biomarkers are potential methods of early detection of melanoma and other types of skin cancer. Earlier diagnosis alone would improve survival, even in the absence of novel therapies.
Metastatic disease accounts for the vast majority of cancer-related deaths. Ensuring a definitive diagnosis and the most effective treatment in a timely fashion is essential for extending life expectancy.
Over the past few decades, the annual incidence of OPSCC has increased sharply in several countries, including in the United States.

Reported results from the coBRIM study have shown that combining the BRAF inhibitor vemurafenib with cobimetinib, a MEK inhibitor, increases progression-free survival (PFS) in patients with BRAF-mutated advanced melanoma compared with vemurafenib alone.

Researchers are finding new ways to diagnose melanoma using machine learning methods.

Cytavis Biopharma GmbH, a Germany-based pharmaceutical company, is developing a natural protein, aviscumine (CY-503), as an immunostimulatory agent to improve response to immunotherapeutic treatment of melanoma.

Amgen and Kite Pharma have announced that they will collaborate on the development of novel CAR T-cell immunotherapies, with Amgen providing cancer targets and Kite offering its engineered autologous cell therapy platform.

The FDA approved nivolumab for patients with advanced melanoma in December 2014, joining 6 other melanoma treatments approved in the past 3 years, including monoclonal antibodies pembrolizumab and ipilimumab.

The FDA has approved nivolumab (Opdivo) for patients with unresectable or metastatic melanoma following treatment with ipilimumab or a BRAF inhibitor, based on data from the phase III CheckMate-037 trial.

The anti-CD19 chimeric antigen receptor (CAR)-modified T-cell therapy CTL019 demonstrated a 92% complete response (CR) rate in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

The programmed death receptor-1 (PD-1) ligand, PD-L1, has become a viable target for immunotherapy in cancer, with multiple antibodies now in development.
Checkpoint inhibition has demonstrated efficacy for the treatment of melanoma in several clinical trials. However, biomarkers to predict safety and efficacy of immunotherapies in individual melanoma patients are lacking.

Scientists are beginning to find that combination therapy improves outcomes for patients, particularly with ipilimumab-nivolumab combination therapy.
As its CAR T cell and high-affinity TCR products continue to advance in clinical trials, Juno Therapeutics, Inc, filed a registration statement for an initial public offering (IPO) of its common stock on November 17.

Knowledge of genetic expression of melanocytic lesions significantly reduced the number of indeterminate diagnoses made by dermatopathologists.

Pembrolizumab (Keytruda) significantly improved progression-free survival (PFS) by over 43% compared with chemotherapy as a treatment for patients with metastatic melanoma who were refractory to ipilimumab (Yervoy).

Frontline treatment with nivolumab (Opdivo) significantly extended overall survival (OS) and progression-free survival (PFS) when compared with dacarbazine in patients with untreated BRAF wild-type advanced melanoma.

Several novel approaches to the delivery of therapeutics for advanced melanoma may improve treatment efficacy and patient survival.

Recent news stories profiling a cancer patient whose last hope rests on treatment by injections of the virus that causes AIDS may have created some misconceptions regarding a new cancer immunotherapy.